Nucleolar Essential Protein 1 (Nep1): Elucidation of enzymatic catalysis mechanism by molecular dynamics simulation and quantum mechanics study
- PMID: 37649713
- PMCID: PMC10462857
- DOI: 10.1016/j.csbj.2023.08.001
Nucleolar Essential Protein 1 (Nep1): Elucidation of enzymatic catalysis mechanism by molecular dynamics simulation and quantum mechanics study
Abstract
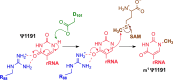
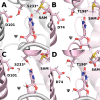
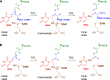
The Nep1 protein is essential for the formation of eukaryotic and archaeal small ribosomal subunits, and it catalyzes the site-directed SAM-dependent methylation of pseudouridine (Ψ) during pre-rRNA processing. It possesses a non-trivial topology, namely, a 31 knot in the active site. Here, we address the issue of seemingly unfeasible deprotonation of Ψ in Nep1 active site by a distant aspartate residue (D101 in S. cerevisiae), using a combination of bioinformatics, computational, and experimental methods. We identified a conserved hydroxyl-containing amino acid (S233 in S. cerevisiae, T198 in A. fulgidus) that may act as a proton-transfer mediator. Molecular dynamics simulations, based on the crystal structure of S. cerevisiae, and on a complex generated by molecular docking in A. fulgidus, confirmed that this amino acid can shuttle protons, however, a water molecule in the active site may also serve this role. Quantum-chemical calculations based on density functional theory and the cluster approach showed that the water-mediated pathway is the most favorable for catalysis. Experimental kinetic and mutational studies reinforce the requirement for the aspartate D101, but not S233. These findings provide insight into the catalytic mechanisms underlying proton transfer over extended distances and comprehensively elucidate the mode of action of Nep1.
Keywords: Enzymatic catalysis; Methylation; Nep1; Proton transfer; RNA processing; Trefoil knot.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
-
- Cantoni G.L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44(1):435–451. - PubMed
-
- Anantharaman V., Koonin E.V., Aravind L. Spout: a class of methyltransferases that includes spou and trmd rna methylase superfamilies, and novel superfamilies of predicted prokaryotic rna methylases. J Mol Microbiol Biotechnol. 2002;4(1):71–76. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
