Polymeric and biological membranes for organ-on-a-chip devices
- PMID: 37649779
- PMCID: PMC10462672
- DOI: 10.1038/s41378-023-00579-z
Polymeric and biological membranes for organ-on-a-chip devices
Abstract
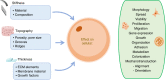
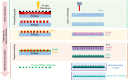
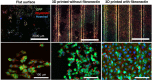
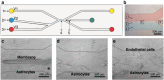
Membranes are fundamental elements within organ-on-a-chip (OOC) platforms, as they provide adherent cells with support, allow nutrients (and other relevant molecules) to permeate/exchange through membrane pores, and enable the delivery of mechanical or chemical stimuli. Through OOC platforms, physiological processes can be studied in vitro, whereas OOC membranes broaden knowledge of how mechanical and chemical cues affect cells and organs. OOCs with membranes are in vitro microfluidic models that are used to replace animal testing for various applications, such as drug discovery and disease modeling. In this review, the relevance of OOCs with membranes is discussed as well as their scaffold and actuation roles, properties (physical and material), and fabrication methods in different organ models. The purpose was to aid readers with membrane selection for the development of OOCs with specific applications in the fields of mechanistic, pathological, and drug testing studies. Mechanical stimulation from liquid flow and cyclic strain, as well as their effects on the cell's increased physiological relevance (IPR), are described in the first section. The review also contains methods to fabricate synthetic and ECM (extracellular matrix) protein membranes, their characteristics (e.g., thickness and porosity, which can be adjusted depending on the application, as shown in the graphical abstract), and the biological materials used for their coatings. The discussion section joins and describes the roles of membranes for different research purposes and their advantages and challenges.
Keywords: Materials science; Microfluidics.
© Aerospace Information Research Institute, Chinese Academy of Sciences 2023.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures





References
-
- Yang X, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18:486–495. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
