Assessment of the proarrhythmic effects of repurposed antimalarials for COVID-19 treatment using a comprehensive in vitro proarrhythmia assay (CiPA)
- PMID: 37649890
- PMCID: PMC10464612
- DOI: 10.3389/fphar.2023.1220796
Assessment of the proarrhythmic effects of repurposed antimalarials for COVID-19 treatment using a comprehensive in vitro proarrhythmia assay (CiPA)
Abstract
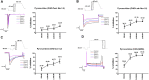
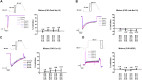
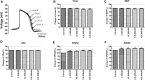
Due to the outbreak of the SARS-CoV-2 virus, drug repurposing and Emergency Use Authorization have been proposed to treat the coronavirus disease 2019 (COVID-19) during the pandemic. While the efficiency of the drugs has been discussed, it was identified that certain compounds, such as chloroquine and hydroxychloroquine, cause QT interval prolongation and potential cardiotoxic effects. Drug-induced cardiotoxicity and QT prolongation may lead to life-threatening arrhythmias such as torsades de pointes (TdP), a potentially fatal arrhythmic symptom. Here, we evaluated the risk of repurposed pyronaridine or artesunate-mediated cardiac arrhythmias alone and in combination for COVID-19 treatment through in vitro and in silico investigations using the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative. The potential effects of each drug or in combinations on cardiac action potential (AP) and ion channels were explored using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and Chinese hamster ovary (CHO) cells transiently expressing cardiac ion channels (Nav1.5, Cav1.2, and hERG). We also performed in silico computer simulation using the optimized O'Hara-Rudy human ventricular myocyte model (ORd model) to classify TdP risk. Artesunate and dihydroartemisinin (DHA), the active metabolite of artesunate, are classified as a low risk of inducing TdP based on the torsade metric score (TMS). Moreover, artesunate does not significantly affect the cardiac APs of hiPSC-CMs even at concentrations up to 100 times the maximum serum concentration (Cmax). DHA modestly prolonged at APD90 (10.16%) at 100 times the Cmax. When considering Cmax, pyronaridine, and the combination of both drugs (pyronaridine and artesunate) are classified as having an intermediate risk of inducing TdP. However, when considering the unbound concentration (the free fraction not bound to carrier proteins or other tissues inducing pharmacological activity), both drugs are classified as having a low risk of inducing TdP. In summary, pyronaridine, artesunate, and a combination of both drugs have been confirmed to pose a low proarrhythmogenic risk at therapeutic and supratherapeutic (up to 4 times) free Cmax. Additionally, the CiPA initiative may be suitable for regulatory use and provide novel insights for evaluating drug-induced cardiotoxicity.
Keywords: COVID-19; CiPA; antimalarials; cardiotoxicity; electrophysiology.
Copyright © 2023 Yoon, Lee, Jeong, Lim, Park and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Authier S., Pugsley M. K., Koerner J. E., Fermini B., Redfern W. S., Valentin J.-P., et al. (2017). Proarrhythmia liability assessment and the comprehensive in vitro proarrhythmia assay (CiPA): an industry survey on current practice. J. Pharmacol. Toxicol. Methods 86, 34–43. 10.1016/j.vascn.2017.02.021 - DOI - PubMed
-
- Bae J.-Y., Lee G. E., Park H., Cho J., Kim Y.-E., Lee J.-Y., et al. (2020). Pyronaridine and artesunate are potential antiviral drugs against COVID-19 and influenza, 2020 2007 2028.225102 bioRxiv. 10.1101/2020.07.28.225102 - DOI
-
- Borba M. G. S., Val F. F. A., Sampaio V. S., Alexandre M. a. A., Melo G. C., Brito M., et al. (2020). Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 3, e208857. 10.1001/jamanetworkopen.2020.8857 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

