This is a preprint.
Personalized chronic adaptive deep brain stimulation outperforms conventional stimulation in Parkinson's disease
- PMID: 37649907
- PMCID: PMC10463549
- DOI: 10.1101/2023.08.03.23293450
Personalized chronic adaptive deep brain stimulation outperforms conventional stimulation in Parkinson's disease
Update in
-
Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson's disease: a blinded randomized feasibility trial.Nat Med. 2024 Nov;30(11):3345-3356. doi: 10.1038/s41591-024-03196-z. Epub 2024 Aug 19. Nat Med. 2024. PMID: 39160351 Free PMC article. Clinical Trial.
Abstract
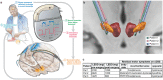
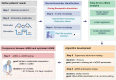
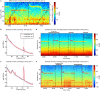
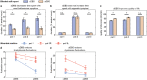
Deep brain stimulation is a widely used therapy for Parkinson's disease (PD) but currently lacks dynamic responsiveness to changing clinical and neural states. Feedback control has the potential to improve therapeutic effectiveness, but optimal control strategy and additional benefits of "adaptive" neurostimulation are unclear. We implemented adaptive subthalamic nucleus stimulation, controlled by subthalamic or cortical signals, in three PD patients (five hemispheres) during normal daily life. We identified neurophysiological biomarkers of residual motor fluctuations using data-driven analyses of field potentials over a wide frequency range and varying stimulation amplitudes. Narrowband gamma oscillations (65-70 Hz) at either site emerged as the best control signal for sensing during stimulation. A blinded, randomized trial demonstrated improved motor symptoms and quality of life compared to clinically optimized standard stimulation. Our approach highlights the promise of personalized adaptive neurostimulation based on data-driven selection of control signals and may be applied to other neurological disorders.
Figures


References
-
- Marceglia S. et al. Deep brain stimulation: is it time to change gears by closing the loop? J. Neural Eng. 18, (2021). - PubMed
-
- Stanslaski S. et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 20, 410–421 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
