Prey species and abundance affect growth and photosynthetic performance of the polyphagous sea slug Elysia crispata
- PMID: 37650060
- PMCID: PMC10465201
- DOI: 10.1098/rsos.230810
Prey species and abundance affect growth and photosynthetic performance of the polyphagous sea slug Elysia crispata
Abstract
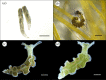
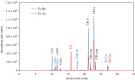
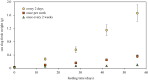
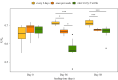
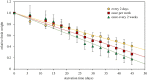
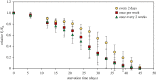
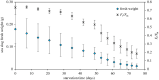
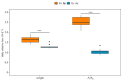
Some sacoglossan sea slugs steal functional macroalgal chloroplasts (kleptoplasts). In this study, we investigated the effects of algal prey species and abundance on the growth and photosynthetic capacity of the tropical polyphagous sea slug Elysia crispata. Recently hatched sea slugs fed and acquired chloroplasts from the macroalga Bryopsis plumosa, but not from Acetabularia acetabulum. However, adult sea slugs were able to switch diet to A. acetabulum, rapidly replacing the great majority of the original kleptoplasts. When fed with B. plumosa, higher feeding frequency resulted in significantly higher growth and kleptoplast photosynthetic yield, as well as a slower relative decrease in these parameters upon starvation. Longevity of A. acetabulum-derived chloroplasts in E. crispata was over twofold that of B. plumosa. Furthermore, significantly lower relative weight loss under starvation was observed in sea slugs previously fed on A. acetabulum than on B. plumosa. This study shows that functionality and longevity of kleptoplasts in photosynthetic sea slugs depend on the origin of the plastids. Furthermore, we have identified A. acetabulum as a donor of photosynthetically efficient chloroplasts common to highly specialized monophagous and polyphagous sea slugs capable of long-term retention, which opens new experimental routes to unravel the unsolved mysteries of kleptoplasty.
Keywords: Acetabularia acetabulum; Bryopsis plumosa; Sacoglossa; chloroplast; kleptoplast retention time; starvation tolerance.
© 2023 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures
Similar articles
-
Food shaped photosynthesis: Photophysiology of the sea slug Elysia viridis fed with two alternative chloroplast donors.Open Res Eur. 2024 Mar 13;3:107. doi: 10.12688/openreseurope.16162.2. eCollection 2023. Open Res Eur. 2024. PMID: 38725452 Free PMC article.
-
Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts.Biology (Basel). 2022 Aug 12;11(8):1207. doi: 10.3390/biology11081207. Biology (Basel). 2022. PMID: 36009836 Free PMC article.
-
A reference genome for the long-term kleptoplast-retaining sea slug Elysia crispata morphotype clarki.G3 (Bethesda). 2023 Dec 6;13(12):jkad234. doi: 10.1093/g3journal/jkad234. G3 (Bethesda). 2023. PMID: 37816307 Free PMC article.
-
Photophysiology of kleptoplasts: photosynthetic use of light by chloroplasts living in animal cells.Philos Trans R Soc Lond B Biol Sci. 2014 Mar 3;369(1640):20130242. doi: 10.1098/rstb.2013.0242. Print 2014 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24591722 Free PMC article. Review.
-
Crawling leaves: photosynthesis in sacoglossan sea slugs.J Exp Bot. 2013 Oct;64(13):3999-4009. doi: 10.1093/jxb/ert197. Epub 2013 Jul 11. J Exp Bot. 2013. PMID: 23846876 Review.
Cited by
-
Habitat shapes the lipidome of the tropical photosynthetic sea slug Elysia crispata.Mar Life Sci Technol. 2025 Apr 7;7(2):382-396. doi: 10.1007/s42995-025-00281-1. eCollection 2025 May. Mar Life Sci Technol. 2025. PMID: 40417258 Free PMC article.
-
Antimicrobial, Antioxidant and Anti-Inflammatory Activities of the Mucus of the Tropical Sea Slug Elysia crispata.Molecules. 2024 Sep 27;29(19):4593. doi: 10.3390/molecules29194593. Molecules. 2024. PMID: 39407523 Free PMC article.
-
Food shaped photosynthesis: Photophysiology of the sea slug Elysia viridis fed with two alternative chloroplast donors.Open Res Eur. 2024 Mar 13;3:107. doi: 10.12688/openreseurope.16162.2. eCollection 2023. Open Res Eur. 2024. PMID: 38725452 Free PMC article.
-
Loss of state transitions in Bryopsidales macroalgae and kleptoplastic sea slugs (Gastropoda, Sacoglossa).Commun Biol. 2025 Jun 5;8(1):869. doi: 10.1038/s42003-025-08305-3. Commun Biol. 2025. PMID: 40473784 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

