Disease modeling and gene correction of LGMDR21 iPSCs elucidates the role of POGLUT1 in skeletal muscle maintenance, regeneration, and the satellite cell niche
- PMID: 37650119
- PMCID: PMC10462830
- DOI: 10.1016/j.omtn.2023.07.037
Disease modeling and gene correction of LGMDR21 iPSCs elucidates the role of POGLUT1 in skeletal muscle maintenance, regeneration, and the satellite cell niche
Abstract
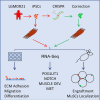
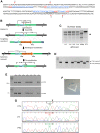
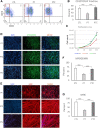
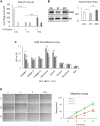
Autosomal recessive limb-girdle muscular dystrophy 21 (LGMDR21) is caused by pathogenic variants in protein O-glucosyltransferase 1 (POGLUT1), which is responsible for O-glucosylation of specific epidermal growth factor (EGF) repeats found in ∼50 mammalian proteins, including Notch receptors. Previous data from patient biopsies indicated that impaired Notch signaling, reduction of muscle stem cells, and accelerated differentiation are probably involved in disease etiopathology. Using patient induced pluripotent stem cells (iPSCs), their corrected isotypes, and control iPSCs, gene expression profiling indicated dysregulation of POGLUT1, NOTCH, muscle development, extracellular matrix (ECM), cell adhesion, and migration as involved pathways. They also exhibited reduced in vitro POGLUT1 enzymatic activity and NOTCH signaling as well as defective myogenesis, proliferation, migration and differentiation. Furthermore, in vivo studies demonstrated significant reductions in engraftment, muscle stem cell formation, PAX7 expression, and maintenance, along with an increased percentage of mislocalized PAX7+ cells in the interstitial space. Gene correction in patient iPSCs using CRISPR-Cas9 nickase led to the rescue of the main in vitro and in vivo phenotypes. These results demonstrate the efficacy of iPSCs and gene correction in disease modeling and rescue of the phenotypes and provide evidence of the involvement of muscle stem cell niche localization, PAX7 expression, and cell migration as possible mechanisms in LGMDR21.
Keywords: CRISPR-Cas9; LGMDR21; MT: RNA/DNA Editing; POGLUT1; gene correction; iPSCs; muscle stem cells; skeletal muscle.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Vissing J. Limb girdle muscular dystrophies: classification, clinical spectrum and emerging therapies. Curr. Opin. Neurol. 2016;29:635–641. - PubMed
-
- Narayanaswami P., Carter G., David W., Weiss M., Amato A.A. Evidence-based guideline summary: Diagnosis and treatment of limb-girdle and distal dystrophies: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2015;84:1720–1721. - PubMed
-
- Magri F., Brajkovic S., Govoni A., Brusa R., Comi G.P. Revised Genetic Classification of Limb Girdle Muscular Dystrophies. Curr. Mol. Med. 2014;14:934–943. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

