Immune Status and SARS-CoV-2 Viral Dynamics
- PMID: 37650232
- PMCID: PMC10469582
- DOI: 10.1093/infdis/jiad200
Immune Status and SARS-CoV-2 Viral Dynamics
Abstract
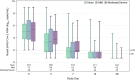
Immunocompromised individuals are disproportionately affected by severe coronavirus disease 2019, but immune compromise is heterogenous, and viral dynamics may vary by the degree of immunosuppression. In this study, we categorized ACTIV-2/A5401 participants based on the extent of immunocompromise into none, mild, moderate, and severe immunocompromise. Moderate/severe immunocompromise was associated with higher nasal viral load at enrollment (adjusted difference in means: 0.47 95% confidence interval, .12-.83 log10 copies/mL) and showed a trend toward higher cumulative nasal RNA levels and plasma viremia compared to nonimmunocompromised individuals. Immunosuppression leads to greater viral shedding and altered severe acute respiratory syndrome coronavirus 2 viral decay kinetics. Clinical Trials Registration. NCT04518410.
Keywords: COVID-19; RNA; SARS-CoV-2; immunocompromise.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. K. W. C. receives research funding from Merck, Sharp & Dohme (paid to institution) and Amgen (research contract with institution); consultancy for Pardes Biosciences; honoraria to the author for continuing medical education presentations (not-for-profit organization) from the International Antiviral Society–USA (IAS-USA); and participation on a data and safety monitoring board (DSMB) or advisory board for the University of California, San Francisco (UCSF) (served as Chair of a safety monitoring committee for an investigator-initiated study where the sponsor is UCSF). E. S. D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV; research support through the institution from Gilead Sciences and GSK/ViiV; participation on a DSMB or advisory board for Gilead and ViiV; and reports support from NIH. D. A. W. has received funding to institution to support research and honoraria for advisory boards and consulting from Gilead Sciences and grant or contracts from Lilly. J. Z. L. has consulted for AbbVie and received a research grant from Merck. J. J. E. is an ad hoc consultant to GSK/Vir Biotechnology and is data monitoring committee chair for Adagio phase 3 studies. J. S. C. has consulted for Merck and Co and reports a leadership or fiduciary role in other board, society, committee, or advocacy groups as a volunteer for the Board of Directors of the IAS-USA and the Foundation Board, Conference on Retroviruses and Opportunistic Infections. D. M. S. has consulted for Bayer Healthcare, Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, and Pharma Holdings; has grants or contracts from NIH (DK131532, AI169609, DA047039, AI036214, AI131385, AI100665, AI126620, funding provided to institution), the James B. Pendleton Charitable Trust John, and the Mary Tu Foundation, including payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events for Medscape (COVID treatment), American Institute continuing medical education (COVID treatment), and Kiadis; stock or stock options for Model Medicine, Linear Therapies, and Cv Biosciences; and receipt of equipment, materials, drugs, medical writings, gifts, or other services from the James B. Pendleton Charitable Trust. C. M. reports participation on a DSMB for BONE STAR. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Hensley MK, Bain WG, Jacobs J, et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis 2021:73:e815–21. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- 3UM1 AI068634-15S1/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- P30 AI152501/AI/NIAID NIH HHS/United States
- T32 AI007387/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1AI068634/NH/NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

