Pooling Different Placebos as a Control Group in a Randomized Platform Trial: Benefits and Challenges From Experience in the ACTIV-2 COVID-19 Trial
- PMID: 37650234
- PMCID: PMC10686688
- DOI: 10.1093/infdis/jiad209
Pooling Different Placebos as a Control Group in a Randomized Platform Trial: Benefits and Challenges From Experience in the ACTIV-2 COVID-19 Trial
Abstract
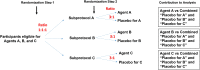
Adaptive platform trials were implemented during the coronavirus disease 2019 (COVID-19) pandemic to rapidly evaluate therapeutics, including the placebo-controlled phase 2/3 ACTIV-2 trial, which studied 7 investigational agents with diverse routes of administration. For each agent, safety and efficacy outcomes were compared to a pooled placebo control group, which included participants who received a placebo for that agent or for other agents in concurrent evaluation. A 2-step randomization framework was implemented to facilitate this. Over the study duration, the pooled placebo design achieved a reduction in sample size of 6% versus a trial involving distinct placebo control groups for evaluating each agent. However, a 26% reduction was achieved during the period when multiple agents were in parallel phase 2 evaluation. We discuss some of the complexities implementing the pooled placebo design versus a design involving nonoverlapping control groups, with the aim of informing the design of future platform trials. Clinical Trials Registration. NCT04518410.
Keywords: COVID-19; adaptive platform trials; pooled placebo; randomization.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. C. B. M. participated on a data safety monitoring board for the BONE STAR study. K. W. C. has received research funding to the institution from Merck Sharp & Dohme; and is a consultant for Pardes Biosciences. E. S. D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV; and research support through the institution from Gilead Sciences and GSK/ViiV. D. A. W. has received funding to the institution to support research and honoraria for advisory boards and consulting from Gilead Sciences. J. S. C. has consulted for Merck and Company. J. J. E. is an ad hoc consultant to GSK/VIR; and data monitoring committee chair for Adagio phase 3 studies. D. M. S. has consulted for Evidera, Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, Arena Pharmaceuticals, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Signant Health, and Brio Clinical. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Adaptive Platform Trials Coalition . Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov 2019; 18:797–807. - PubMed
-
- Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 2015; 313:1619–20. - PubMed
-
- Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol 2020; 125:1–8. - PubMed
-
- Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017; 377:62–70. - PubMed
-
- Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016; 375:65–74. - PubMed

