Long COVID After Bamlanivimab Treatment
- PMID: 37650236
- PMCID: PMC10686694
- DOI: 10.1093/infdis/jiad286
Long COVID After Bamlanivimab Treatment
Abstract
Background: Prospective evaluations of long COVID in outpatients with coronavirus disease 2019 (COVID-19) are lacking. We aimed to determine the frequency and predictors of long COVID after treatment with the monoclonal antibody bamlanivimab in ACTIV-2/A5401.
Methods: Data were analyzed from participants who received bamlanivimab 700 mg in ACTIV-2 from October 2020 to February 2021. Long COVID was defined as the presence of self-assessed COVID symptoms at week 24. Self-assessed return to pre-COVID health was also examined. Associations were assessed by regression models.
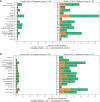
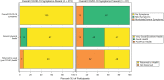
Results: Among 506 participants, median age was 51 years. Half were female, 5% Black/African American, and 36% Hispanic/Latino. At 24 weeks, 18% reported long COVID and 15% had not returned to pre-COVID health. Smoking (adjusted risk ratio [aRR], 2.41 [95% confidence interval {CI}, 1.34- 4.32]), female sex (aRR, 1.91 [95% CI, 1.28-2.85]), non-Hispanic ethnicity (aRR, 1.92 [95% CI, 1.19-3.13]), and presence of symptoms 22-28 days posttreatment (aRR, 2.70 [95% CI, 1.63-4.46]) were associated with long COVID, but nasal severe acute respiratory syndrome coronavirus 2 RNA was not.
Conclusions: Long COVID occurred despite early, effective monoclonal antibody therapy and was associated with smoking, female sex, and non-Hispanic ethnicity, but not viral burden. The strong association between symptoms 22-28 days after treatment and long COVID suggests that processes of long COVID start early and may need early intervention.
Clinical trials registration: NCT04518410.
Keywords: bamlanivimab; clinical trial; long COVID; postacute sequelae of SARS-CoV-2 infection (PASC); symptom.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. T. H. E. serves as a consultant for Tonix Pharmaceuticals. N. J. received salary support to the institution from Sagent Pharmaceuticals. D. A. W. has received funding to his institution to support research and honoraria for advisory boards and consulting from Gilead Sciences. E. S. D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV and research support through his institution from Gilead Sciences and GSK/ViiV. J. Z. L. has received research funding from Merck. P. K. is an employee and shareholder of Eli Lilly. J.J.E. is an ad hoc consultant to GSK/VIR, data monitoring committee chair for Adagio Phase 3 studies. J. S. C. has consulted for Merck and Company. D. M. S. has consulted for Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, Arena Pharmaceuticals, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Signant Health, and Brio Clinical. K. W. C. received research funding to institution from Merck Sharp & Dohme and is a consultant for Pardes Biosciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

