Validity and Characterization of Time to Symptom Resolution Outcome Measures in the ACTIV-2/A5401 Outpatient COVID-19 Treatment Trial
- PMID: 37650237
- PMCID: PMC10469584
- DOI: 10.1093/infdis/jiad300
Validity and Characterization of Time to Symptom Resolution Outcome Measures in the ACTIV-2/A5401 Outpatient COVID-19 Treatment Trial
Abstract
Background: Time to symptom resolution measures were used in outpatient coronavirus disease 2019 (COVID-19) treatment trials without prior validation.
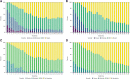
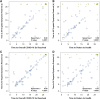
Methods: ACTIV-2/A5401 trial participants completed a COVID-19 diary assessing 13 targeted symptoms and global experience (overall COVID-19 symptoms, return to pre-COVID-19 health) daily for 29 days. We evaluated concordance of time to sustained (2 days) resolution of all targeted symptoms (TSR) with resolution of overall symptoms and return to health in participants receiving placebo.
Results: The analysis included 77 high-risk and 81 standard-risk participants with overall median 6 days of symptoms at entry and median age 47 years, 50% female, 82% white, and 31% Hispanic/Latino. Correlation between TSR and resolution of overall symptoms was 0.80 and 0.68, and TSR and return to health, 0.66 and 0.57 for high- and standard-risk groups, respectively. Of the high- and standard-risk participants, 61% and 79%, respectively, achieved targeted symptom resolution, of which 47% and 43%, respectively, reported symptom recurrence. Requiring >2 days to define sustained resolution reduced the frequency of recurrences.
Conclusions: There was good internal consistency between TSR and COVID-19-specific global outcomes, supporting TSR as a trial end point. Requiring >2 days of symptom resolution better addresses natural symptom fluctuations but must be balanced against the potential influence of non-COVID-19 symptoms.
Clinical trials registration: NCT04518410.
Keywords: ACTIV-2; COVID-19; internal validity; patient-reported outcomes; symptom diary; symptom outcomes; symptom rebound; symptom recurrence; time to symptom resolution.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . K. W. C. reports research funding to the institution from Merck Sharp & Dohme; and is a consultant for Pardes Biosciences. D. A. W. has received funding to the institution to support research; and honoraria for advisory boards and consulting from Gilead Sciences. E. S. D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV; and research support through the institution from Gilead Sciences and GSK/ViiV. J. J. E. is an ad hoc consultant to GSK/VIR; and data monitoring committee chair for Adagio phase 3 studies. J. S. C. has consulted for Merck and Company. D. M. S. has consulted for Evidera, Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, Arena Pharmaceuticals, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Signant Health, and Brio Clinical. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures