Src tyrosine kinase promotes cardiac remodeling induced by chronic sympathetic activation
- PMID: 37650260
- PMCID: PMC10611920
- DOI: 10.1042/BSR20231097
Src tyrosine kinase promotes cardiac remodeling induced by chronic sympathetic activation
Abstract
Cardiac remodeling serves as the underlying pathological basis for numerous cardiovascular diseases and represents a pivotal stage for intervention. The excessive activation of β-adrenergic receptors (β-ARs) assumes a crucial role in cardiac remodeling. Nonetheless, the underlying molecular mechanisms governing β-AR-induced cardiac remodeling remain largely unresolved. In the present study, we identified Src tyrosine kinase as a key player in the cardiac remodeling triggered by excessive β-AR activation. Our findings demonstrated that Src mediates isoproterenol (ISO)-induced cardiac hypertrophy, fibrosis, and inflammation in vivo. Furthermore, Src facilitates β-AR-mediated proliferation and transdifferentiation of cardiac fibroblasts, and hypertrophy and cardiomyocytes in vitro. Subsequent investigations have substantiated that Src mediates β-AR induced the extracellular signal-regulated protein kinase (ERK1/2) signaling pathway activated by β-AR. Our research presents compelling evidence that Src promotes β-AR-induced cardiac remodeling in both in vivo and in vitro settings. It establishes the promoting effect of the β-AR/Src/ERK signaling pathway on overall cardiac remodeling in cardiac fibroblasts and underscores the potential of Src as a therapeutic target for cardiac remodeling.
Keywords: Src; cardiac remodeling; cardiomyocyte; β-adrenergic receptor.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


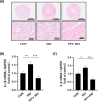
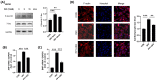
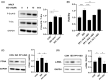
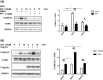
Similar articles
-
Endocytosis machinery is required for beta1-adrenergic receptor-induced hypertrophy in neonatal rat cardiac myocytes.Cardiovasc Res. 2008 Apr 1;78(1):36-44. doi: 10.1093/cvr/cvn008. Epub 2008 Jan 14. Cardiovasc Res. 2008. PMID: 18194989 Free PMC article.
-
Beta-adrenergic receptor-mediated DNA synthesis in cardiac fibroblasts is dependent on transactivation of the epidermal growth factor receptor and subsequent activation of extracellular signal-regulated kinases.J Biol Chem. 2002 Aug 30;277(35):32116-23. doi: 10.1074/jbc.M204895200. Epub 2002 Jun 4. J Biol Chem. 2002. PMID: 12048215
-
Src is required for mechanical stretch-induced cardiomyocyte hypertrophy through angiotensin II type 1 receptor-dependent β-arrestin2 pathways.PLoS One. 2014 Apr 3;9(4):e92926. doi: 10.1371/journal.pone.0092926. eCollection 2014. PLoS One. 2014. PMID: 24699426 Free PMC article.
-
Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance.Sheng Li Xue Bao. 2004 Feb 25;56(1):1-15. Sheng Li Xue Bao. 2004. PMID: 14985822 Review.
-
Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts.Circ Res. 1998 Feb 23;82(3):337-45. doi: 10.1161/01.res.82.3.337. Circ Res. 1998. PMID: 9486662 Review.
Cited by
-
Miglustat ameliorates isoproterenol-induced cardiac fibrosis via targeting UGCG.Mol Med. 2025 Feb 11;31(1):55. doi: 10.1186/s10020-025-01093-w. Mol Med. 2025. PMID: 39934657 Free PMC article.
-
DIGGER 2.0: digging into the functional impact of differential splicing on human and mouse disorders.Nucleic Acids Res. 2025 Jul 7;53(W1):W245-W252. doi: 10.1093/nar/gkaf384. Nucleic Acids Res. 2025. PMID: 40337913 Free PMC article.
-
Tyrosine phosphorylation of Kir6.2 subunit negatively regulates cardiac KATP channel activity.Basic Res Cardiol. 2025 Jun;120(3):473-488. doi: 10.1007/s00395-025-01108-x. Epub 2025 Apr 19. Basic Res Cardiol. 2025. PMID: 40251281 Free PMC article.
References
-
- Savarese G., Bodegard J., Norhammar A., Sartipy P., Thuresson M., Cowie M.R.et al. . (2021) Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur. J. Heart Fail. 23, 1499–1511, 10.1002/ejhf.2271 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

