The kleisin subunit controls the function of C. elegans meiotic cohesins by determining the mode of DNA binding and differential regulation by SCC-2 and WAPL-1
- PMID: 37650378
- PMCID: PMC10497282
- DOI: 10.7554/eLife.84138
The kleisin subunit controls the function of C. elegans meiotic cohesins by determining the mode of DNA binding and differential regulation by SCC-2 and WAPL-1
Abstract
The cohesin complex plays essential roles in chromosome segregation, 3D genome organisation, and DNA damage repair through its ability to modify DNA topology. In higher eukaryotes, meiotic chromosome function, and therefore fertility, requires cohesin complexes containing meiosis-specific kleisin subunits: REC8 and RAD21L in mammals and REC-8 and COH-3/4 in Caenorhabditis elegans. How these complexes perform the multiple functions of cohesin during meiosis and whether this involves different modes of DNA binding or dynamic association with chromosomes is poorly understood. Combining time-resolved methods of protein removal with live imaging and exploiting the temporospatial organisation of the C. elegans germline, we show that REC-8 complexes provide sister chromatid cohesion (SCC) and DNA repair, while COH-3/4 complexes control higher-order chromosome structure. High-abundance COH-3/4 complexes associate dynamically with individual chromatids in a manner dependent on cohesin loading (SCC-2) and removal (WAPL-1) factors. In contrast, low-abundance REC-8 complexes associate stably with chromosomes, tethering sister chromatids from S-phase until the meiotic divisions. Our results reveal that kleisin identity determines the function of meiotic cohesin by controlling the mode and regulation of cohesin-DNA association, and are consistent with a model in which SCC and DNA looping are performed by variant cohesin complexes that coexist on chromosomes.
Keywords: C. elegans; SCC-2; WAPL; cell biology; chromosomes; cohesin; gene expression; meiosis; sister chromatid cohesion.
© 2023, Castellano-Pozo et al.
Conflict of interest statement
MC, GS, CB, JP, PL, JD, AJ, OC, NS, JP, EM No competing interests declared
Figures



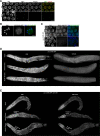
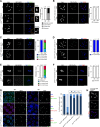


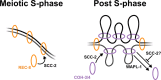
Update of
References
-
- Burkhardt S, Borsos M, Szydlowska A, Godwin J, Williams SA, Cohen PE, Hirota T, Saitou M, Tachibana-Konwalski K. Chromosome cohesion established by Rec8-Cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Current Biology. 2016;26:678–685. doi: 10.1016/j.cub.2015.12.073. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

