Optimisation of TP53 reporters by systematic dissection of synthetic TP53 response elements
- PMID: 37650627
- PMCID: PMC10570033
- DOI: 10.1093/nar/gkad718
Optimisation of TP53 reporters by systematic dissection of synthetic TP53 response elements
Abstract
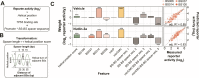
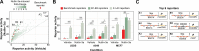
TP53 is a transcription factor that controls multiple cellular processes, including cell cycle arrest, DNA repair and apoptosis. The relation between TP53 binding site architecture and transcriptional output is still not fully understood. Here, we systematically examined in three different cell lines the effects of binding site affinity and copy number on TP53-dependent transcriptional output, and also probed the impact of spacer length and sequence between adjacent binding sites, and of core promoter identity. Paradoxically, we found that high-affinity TP53 binding sites are less potent than medium-affinity sites. TP53 achieves supra-additive transcriptional activation through optimally spaced adjacent binding sites, suggesting a cooperative mechanism. Optimally spaced adjacent binding sites have a ∼10-bp periodicity, suggesting a role for spatial orientation along the DNA double helix. We leveraged these insights to construct a log-linear model that explains activity from sequence features, and to identify new highly active and sensitive TP53 reporters.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
Noncanonical DNA motifs as transactivation targets by wild type and mutant p53.PLoS Genet. 2008 Jun 27;4(6):e1000104. doi: 10.1371/journal.pgen.1000104. PLoS Genet. 2008. PMID: 18714371 Free PMC article.
-
Influence of promoter DNA topology on sequence-specific DNA binding and transactivation by tumor suppressor p53.Oncogene. 1999 Dec 2;18(51):7310-8. doi: 10.1038/sj.onc.1203139. Oncogene. 1999. PMID: 10602486
-
Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage.Mol Cell Biol. 2007 Dec;27(24):8480-91. doi: 10.1128/MCB.01126-07. Epub 2007 Oct 15. Mol Cell Biol. 2007. PMID: 17938203 Free PMC article.
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
-
Transcriptional regulation by p53: one protein, many possibilities.Cell Death Differ. 2006 Jun;13(6):951-61. doi: 10.1038/sj.cdd.4401916. Cell Death Differ. 2006. PMID: 16575405 Review.
Cited by
-
Determinants of p53 DNA binding, gene regulation, and cell fate decisions.Cell Death Differ. 2024 Jul;31(7):836-843. doi: 10.1038/s41418-024-01326-1. Epub 2024 Jun 29. Cell Death Differ. 2024. PMID: 38951700 Free PMC article. Review.
-
Crosstalk between paralogs and isoforms influences p63-dependent regulatory element activity.Nucleic Acids Res. 2024 Dec 11;52(22):13812-13831. doi: 10.1093/nar/gkae1143. Nucleic Acids Res. 2024. PMID: 39565223 Free PMC article.
-
Optimized reporters for multiplexed detection of transcription factor activity.bioRxiv [Preprint]. 2024 Jul 26:2024.07.26.605239. doi: 10.1101/2024.07.26.605239. bioRxiv. 2024. Update in: Cell Syst. 2024 Dec 18;15(12):1107-1122.e7. doi: 10.1016/j.cels.2024.11.003. PMID: 39091757 Free PMC article. Updated. Preprint.
-
Optimized reporters for multiplexed detection of transcription factor activity.Cell Syst. 2024 Dec 18;15(12):1107-1122.e7. doi: 10.1016/j.cels.2024.11.003. Epub 2024 Dec 6. Cell Syst. 2024. PMID: 39644900 Free PMC article.
-
Context dependent activity of p63-bound gene regulatory elements.bioRxiv [Preprint]. 2024 May 12:2024.05.09.593326. doi: 10.1101/2024.05.09.593326. bioRxiv. 2024. PMID: 38766006 Free PMC article. Preprint.
References
-
- el-Deiry W.S., Kern S.E., Pietenpol J.A., Kinzler K.W., Vogelstein B.. Definition of a consensus binding site for p53. Nat. Genet. 1992; 1:45–49. - PubMed
-
- Ciribilli Y., Monti P., Bisio A., Nguyen H.T., Ethayathulla A.S., Ramos A., Foggetti G., Menichini P., Menendez D., Resnick M.A.et al. .. Transactivation specificity is conserved among p53 family proteins and depends on a response element sequence code. Nucleic Acids Res. 2013; 41:8637–8653. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

