Targeted delivery of a short antimicrobial peptide (CM11) against Helicobacter pylori gastric infection using concanavalin A-coated chitosan nanoparticles
- PMID: 37650975
- PMCID: PMC10471652
- DOI: 10.1007/s10856-023-06748-w
Targeted delivery of a short antimicrobial peptide (CM11) against Helicobacter pylori gastric infection using concanavalin A-coated chitosan nanoparticles
Abstract
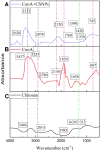
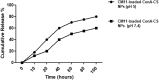
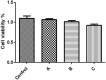
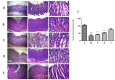
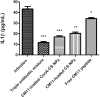
Helicobacter pylori is the cause of most cases of stomach ulcers and also causes some digestive cancers. The emergence and spread of antibiotic-resistant strains of H. pylori is one of the most important challenges in the treatment of its infections. The present study aims to develop a concanavalin A (ConA) coated chitosan (CS) nanocarrier-based drug delivery for the targeted release of peptides to the site of H. pylori infection. Accordingly, chitosan was used as an encapsulating agent for CM11 peptide delivery by applying ionotropic gelation method. Con-A was used for coating CS nanoparticles to target H. pylori. The CS NPs and ConA-CS NPs were characterized by FTIR, dynamic light scattering (DLS), and scanning electron microscopy (SEM). The MIC of CM11-loaded ConA-CS NPs against H. pylori SS1 strain was analyzed in vitro. In order to evaluate the treatment efficiency in vivo, a gastric infection model of H. pylori SS1 strain was established in mice and histopathological studies and IL-1β cytokine assay were performed. Based on the results, the size frequency for CS NPs and ConA-CS NPs was about 200 and 350 nm, respectively. The prepared CM11-loaded ConA-CS NPs exhibited antibacterial activity against H. pylori SS1 strain with a concentration of 32 µg/ml. The highest healing process was observed in synthesized CM11-loaded ConA-CS NPs treatments and a significant decrease in IL-1β was observed. Our findings highlight the potential of chitosan nanoparticles as a drug delivery vehicle in the treatment of gastric infection model of H. pylori SS1 strain.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Zahmatkesh ME, Jahanbakhsh M, Hoseini N, Shegefti S, Peymani A, Dabin H, et al. Effects of exosomes derived from helicobacter pylori outer membrane vesicle-infected hepatocytes on hepatic stellate cell activation and liver fibrosis induction. Front Cell Infect Microbiol. 2022;12:857570. doi: 10.3389/fcimb.2022.857570. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

