Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial
- PMID: 37651119
- PMCID: PMC10472268
- DOI: 10.1001/jama.2023.14530
Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial
Erratum in
-
Error in Byline.JAMA. 2024 Feb 27;331(8):710. doi: 10.1001/jama.2024.0828. JAMA. 2024. PMID: 38277159 Free PMC article. No abstract available.
Abstract
Importance: Psilocybin shows promise as a treatment for major depressive disorder (MDD).
Objective: To evaluate the magnitude, timing, and durability of antidepressant effects and safety of a single dose of psilocybin in patients with MDD.
Design, setting, and participants: In this phase 2 trial conducted between December 2019 and June 2022 at 11 research sites in the US, participants were randomized in a 1:1 ratio to receive a single dose of psilocybin vs niacin placebo administered with psychological support. Participants were adults aged 21 to 65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition diagnosis of MDD of at least 60 days' duration and moderate or greater symptom severity. Exclusion criteria included history of psychosis or mania, active substance use disorder, and active suicidal ideation with intent. Participants taking psychotropic agents who otherwise met inclusion/exclusion criteria were eligible following medication taper. Primary and secondary outcomes and adverse events (AEs) were assessed at baseline (conducted within 7 days before dosing) and at 2, 8, 15, 29, and 43 days after dosing.
Interventions: Interventions were a 25-mg dose of synthetic psilocybin or a 100-mg dose of niacin in identical-appearing capsules, each administered with psychological support.
Main outcomes and measures: The primary outcome was change in central rater-assessed Montgomery-Asberg Depression Rating Scale (MADRS) score (range, 0-60; higher scores indicate more severe depression) from baseline to day 43. The key secondary outcome measure was change in MADRS score from baseline to day 8. Other secondary outcomes were change in Sheehan Disability Scale score from baseline to day 43 and MADRS-defined sustained response and remission. Participants, study site personnel, study sponsor, outcome assessors (raters), and statisticians were blinded to treatment assignment.
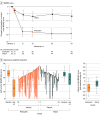
Results: A total of 104 participants (mean [SD] age, 41.1 [11.3] years; 52 [50%] women) were randomized (51 to the psilocybin group and 53 to the niacin group). Psilocybin treatment was associated with significantly reduced MADRS scores compared with niacin from baseline to day 43 (mean difference,-12.3 [95% CI, -17.5 to -7.2]; P <.001) and from baseline to day 8 (mean difference, -12.0 [95% CI, -16.6 to -7.4]; P < .001). Psilocybin treatment was also associated with significantly reduced Sheehan Disability Scale scores compared with niacin (mean difference, -2.31 [95% CI, 3.50-1.11]; P < .001) from baseline to day 43. More participants receiving psilocybin had sustained response (but not remission) than those receiving niacin. There were no serious treatment-emergent AEs; however, psilocybin treatment was associated with a higher rate of overall AEs and a higher rate of severe AEs.
Conclusions and relevance: Psilocybin treatment was associated with a clinically significant sustained reduction in depressive symptoms and functional disability, without serious adverse events. These findings add to increasing evidence that psilocybin-when administered with psychological support-may hold promise as a novel intervention for MDD.
Trial registration: ClinicalTrials.gov Identifier: NCT03866174.
Conflict of interest statement
Figures
Comment in
-
Psychedelic Therapy-A New Paradigm of Care for Mental Health.JAMA. 2023 Sep 5;330(9):813-814. doi: 10.1001/jama.2023.12900. JAMA. 2023. PMID: 37651148 No abstract available.
References
-
- Almohammed OA, Alsalem AA, Almangour AA, Alotaibi LH, Al Yami MS, Lai L. Antidepressants and health-related quality of life (HRQoL) for patients with depression: analysis of the medical expenditure panel survey from the United States. PLoS One. 2022;17(4):e0265928. doi: 10.1371/journal.pone.0265928 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

