The unfolded protein response links ER stress to cancer-associated thrombosis
- PMID: 37651191
- PMCID: PMC10629814
- DOI: 10.1172/jci.insight.170148
The unfolded protein response links ER stress to cancer-associated thrombosis
Abstract
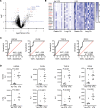
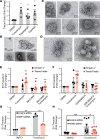
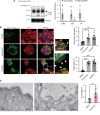
Thrombosis is a common complication of advanced cancer, yet the cellular mechanisms linking malignancy to thrombosis are poorly understood. The unfolded protein response (UPR) is an ER stress response associated with advanced cancers. A proteomic evaluation of plasma from patients with gastric and non-small cell lung cancer who were monitored prospectively for venous thromboembolism demonstrated increased levels of UPR-related markers in plasma of patients who developed clots compared with those who did not. Release of procoagulant activity into supernatants of gastric, lung, and pancreatic cancer cells was enhanced by UPR induction and blocked by antagonists of the UPR receptors inositol-requiring enzyme 1α (IRE1α) and protein kinase RNA-like endoplasmic reticulum kinase (PERK). Release of extracellular vesicles bearing tissue factor (EVTFs) from pancreatic cancer cells was inhibited by siRNA-mediated knockdown of IRE1α/XBP1 or PERK pathways. Induction of UPR did not increase tissue factor (TF) synthesis, but rather stimulated localization of TF to the cell surface. UPR-induced TF delivery to EVTFs was inhibited by ADP-ribosylation factor 1 knockdown or GBF1 antagonism, verifying the role of vesicular trafficking. Our findings show that UPR activation resulted in increased vesicular trafficking leading to release of prothrombotic EVTFs, thus providing a mechanistic link between ER stress and cancer-associated thrombosis.
Keywords: Cancer; Hematology; Thrombosis.
Conflict of interest statement
Figures




Similar articles
-
The endoplasmic reticulum stress sensor IRE1α protects cells from apoptosis induced by the coronavirus infectious bronchitis virus.J Virol. 2014 Nov;88(21):12752-64. doi: 10.1128/JVI.02138-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142592 Free PMC article.
-
Profiling of Unfolded Protein Response Markers and Effect of IRE1α-specific Inhibitor in Pituitary Neuroendocrine Tumor.Endocrinology. 2024 Feb 20;165(4):bqae008. doi: 10.1210/endocr/bqae008. Endocrinology. 2024. PMID: 38289718
-
Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR).Int J Mol Sci. 2022 Dec 29;24(1):577. doi: 10.3390/ijms24010577. Int J Mol Sci. 2022. PMID: 36614019 Free PMC article. Review.
-
PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1α-mediated unfolded protein response.Nat Cell Biol. 2012 Nov;14(11):1223-30. doi: 10.1038/ncb2593. Epub 2012 Oct 28. Nat Cell Biol. 2012. PMID: 23103912 Free PMC article.
-
Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review.
Cited by
-
The Importance of Extracellular Vesicle Screening in Gastric Cancer: A 2024 Update.Cancers (Basel). 2024 Jul 18;16(14):2574. doi: 10.3390/cancers16142574. Cancers (Basel). 2024. PMID: 39061213 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

