Endothelial VEGFR2-PLCγ signaling regulates vascular permeability and antitumor immunity through eNOS/Src
- PMID: 37651195
- PMCID: PMC10575733
- DOI: 10.1172/JCI161366
Endothelial VEGFR2-PLCγ signaling regulates vascular permeability and antitumor immunity through eNOS/Src
Abstract
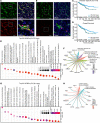
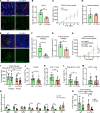
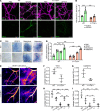
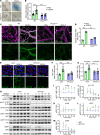
Endothelial phospholipase Cγ (PLCγ) is essential for vascular development; however, its role in healthy, mature, or pathological vessels is unexplored. Here, we show that PLCγ was prominently expressed in vessels of several human cancer forms, notably in renal cell carcinoma (RCC). High PLCγ expression in clear cell RCC correlated with angiogenic activity and poor prognosis, while low expression correlated with immune cell activation. PLCγ was induced downstream of vascular endothelial growth factor receptor 2 (VEGFR2) phosphosite Y1173 (pY1173). Heterozygous Vegfr2Y1173F/+ mice or mice lacking endothelial PLCγ (Plcg1iECKO) exhibited a stabilized endothelial barrier and diminished vascular leakage. Barrier stabilization was accompanied by decreased expression of immunosuppressive cytokines, reduced infiltration of B cells, helper T cells and regulatory T cells, and improved response to chemo- and immunotherapy. Mechanistically, pY1173/PLCγ signaling induced Ca2+/protein kinase C-dependent activation of endothelial nitric oxide synthase (eNOS), required for tyrosine nitration and activation of Src. Src-induced phosphorylation of VE-cadherin at Y685 was accompanied by disintegration of endothelial junctions. This pY1173/PLCγ/eNOS/Src pathway was detected in both healthy and tumor vessels in Vegfr2Y1173F/+ mice, which displayed decreased activation of PLCγ and eNOS and suppressed vascular leakage. Thus, we believe that we have identified a clinically relevant endothelial PLCγ pathway downstream of VEGFR2 pY1173, which destabilizes the endothelial barrier and results in loss of antitumor immunity.
Keywords: Oncology; Signal transduction; Vascular Biology.
Figures
Similar articles
-
eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin.Elife. 2021 Apr 28;10:e64944. doi: 10.7554/eLife.64944. Elife. 2021. PMID: 33908348 Free PMC article.
-
eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases.J Cell Sci. 2013 Dec 15;126(Pt 24):5541-52. doi: 10.1242/jcs.115972. Epub 2013 Sep 17. J Cell Sci. 2013. PMID: 24046447 Free PMC article.
-
The impact of the receptor binding profiles of the vascular endothelial growth factors on their angiogenic features.Biochim Biophys Acta. 2014 Jan;1840(1):454-63. doi: 10.1016/j.bbagen.2013.10.005. Epub 2013 Oct 8. Biochim Biophys Acta. 2014. PMID: 24112971
-
VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd.J Exp Med. 2012 Jul 2;209(7):1363-77. doi: 10.1084/jem.20111343. Epub 2012 Jun 11. J Exp Med. 2012. PMID: 22689825 Free PMC article.
-
Dysfunction of phospholipase Cγ in immune disorders and cancer.Trends Biochem Sci. 2014 Dec;39(12):603-11. doi: 10.1016/j.tibs.2014.09.004. Epub 2014 Oct 30. Trends Biochem Sci. 2014. PMID: 25456276 Review.
Cited by
-
Proteoglycans of basement membranes: Crucial controllers of angiogenesis, neurogenesis, and autophagy.Proteoglycan Res. 2024 Jul-Sep;2(3):e22. doi: 10.1002/pgr2.22. Epub 2024 Jun 29. Proteoglycan Res. 2024. PMID: 39184370 Free PMC article.
-
The impact of bevacizumab intraperitoneal perfusion combined with paclitaxel and platinum-based chemotherapy on serum stromal-derived factor-1α (SDF-1α) and chemokine ligand 5 (CXCL-5) levels in patients with ovarian cancer after tumor cell debulking surgery.Anticancer Drugs. 2025 Mar 1;36(3):232-237. doi: 10.1097/CAD.0000000000001663. Epub 2025 Jan 6. Anticancer Drugs. 2025. PMID: 39757759 Free PMC article.
-
Molecular Mechanisms Regulating Vascular Endothelial Permeability.Int J Mol Sci. 2024 Jun 11;25(12):6415. doi: 10.3390/ijms25126415. Int J Mol Sci. 2024. PMID: 38928121 Free PMC article. Review.
-
Inhibition of Endothelial HIF-1α by IDF-11774 Attenuates Retinal Neovascularization and Vascular Leakage.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):47. doi: 10.1167/iovs.66.9.47. Invest Ophthalmol Vis Sci. 2025. PMID: 40668060 Free PMC article.
-
Hypersensitive blood vessels in Clarkson disease.J Clin Invest. 2024 May 15;134(10):e180795. doi: 10.1172/JCI180795. J Clin Invest. 2024. PMID: 38747291 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

