Differential Proteome Profiling Analysis under Pesticide Stress by the Use of a Nano-UHPLC-MS/MS Untargeted Proteomic-Based Approach on a 3D-Developed Neurospheroid Model: Identification of Protein Interactions, Prognostic Biomarkers, and Potential Therapeutic Targets in Human IDH Mutant High-Grade Gliomas
- PMID: 37651309
- PMCID: PMC10629271
- DOI: 10.1021/acs.jproteome.3c00395
Differential Proteome Profiling Analysis under Pesticide Stress by the Use of a Nano-UHPLC-MS/MS Untargeted Proteomic-Based Approach on a 3D-Developed Neurospheroid Model: Identification of Protein Interactions, Prognostic Biomarkers, and Potential Therapeutic Targets in Human IDH Mutant High-Grade Gliomas
Abstract
High-grade gliomas represent the most common group of infiltrative primary brain tumors in adults associated with high invasiveness, agressivity, and resistance to therapy, which highlights the need to develop potent drugs with novel mechanisms of action. The aim of this study is to reveal changes in proteome profiles under stressful conditions to identify prognostic biomarkers and altered apoptogenic pathways involved in the anticancer action of human isocitrate dehydrogenase (IDH) mutant high-grade gliomas. Our protocol consists first of a 3D in vitro developing neurospheroid model and then treatment by a pesticide mixture at relevant concentrations. Furthermore, we adopted an untargeted proteomic-based approach with high-resolution mass spectrometry for a comparative analysis of the differentially expressed proteins between treated and nontreated spheroids. Our analysis revealed that the majority of altered proteins were key members in glioma pathogenesis, implicated in the cellular metabolism, biological regulation, binding, and catalytic and structural activity and linked to many cascading regulatory pathways. Our finding revealed that grade-IV astrocytomas promote the downstream of the mitogen-activated-protein-kinases/extracellular-signal-regulated kinase (MAPK1/ERK2) pathway involving massive calcium influx. The gonadotrophin-releasing-hormone signaling enhances MAKP activity and may serve as a negative feedback compensating regulator. Thus, our study can pave the way for effective new therapeutic and diagnostic strategies to improve the overall survival.
Keywords: 3D neurospheroids; PPI networks; apoptogenic pathways; bioinformatic analysis; gene ontology; grade-IV astrocytomas IDH mutant; nanoflow UHPLC; neurotoxicity; proteomics; tandem mass spectrometry (MS/MS).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


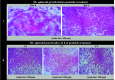
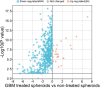

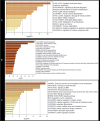

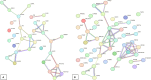

References
-
- Friedman H. S.; Kerby T.; Calvert H. Temozolomide and treatment of malignant glioma. Clin. Canc. Res. 2000, 6, 2585–2597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

