Phylogenetic inference of the emergence of sequence modules and protein-protein interactions in the ADAMTS-TSL family
- PMID: 37651409
- PMCID: PMC10499240
- DOI: 10.1371/journal.pcbi.1011404
Phylogenetic inference of the emergence of sequence modules and protein-protein interactions in the ADAMTS-TSL family
Abstract
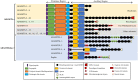
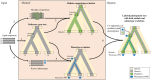
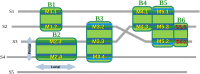
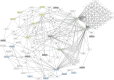
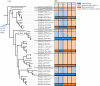
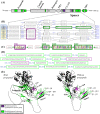
Numerous computational methods based on sequences or structures have been developed for the characterization of protein function, but they are still unsatisfactory to deal with the multiple functions of multi-domain protein families. Here we propose an original approach based on 1) the detection of conserved sequence modules using partial local multiple alignment, 2) the phylogenetic inference of species/genes/modules/functions evolutionary histories, and 3) the identification of co-appearances of modules and functions. Applying our framework to the multidomain ADAMTS-TSL family including ADAMTS (A Disintegrin-like and Metalloproteinase with ThromboSpondin motif) and ADAMTS-like proteins over nine species including human, we identify 45 sequence module signatures that are associated with the occurrence of 278 Protein-Protein Interactions in ancestral genes. Some of these signatures are supported by published experimental data and the others provide new insights (e.g. ADAMTS-5). The module signatures of ADAMTS ancestors notably highlight the dual variability of the propeptide and ancillary regions suggesting the importance of these two regions in the specialization of ADAMTS during evolution. Our analyses further indicate convergent interactions of ADAMTS with COMP and CCN2 proteins. Overall, our study provides 186 sequence module signatures that discriminate distinct subgroups of ADAMTS and ADAMTSL and that may result from selective pressures on novel functions and phenotypes.
Copyright: © 2023 Dennler et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
The evolutionary conservation of the A Disintegrin-like and Metalloproteinase domain with Thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish.BMC Evol Biol. 2015 Feb 15;15:22. doi: 10.1186/s12862-015-0281-9. BMC Evol Biol. 2015. PMID: 25879701 Free PMC article.
-
A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms.J Biol Chem. 2009 Nov 13;284(46):31493-7. doi: 10.1074/jbc.R109.052340. Epub 2009 Sep 4. J Biol Chem. 2009. PMID: 19734141 Free PMC article. Review.
-
Identification and characterization of ADAMTS-20 defines a novel subfamily of metalloproteinases-disintegrins with multiple thrombospondin-1 repeats and a unique GON domain.J Biol Chem. 2003 Apr 11;278(15):13382-9. doi: 10.1074/jbc.M211900200. Epub 2003 Jan 31. J Biol Chem. 2003. PMID: 12562771
-
Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains.Gene. 2002 Jan 23;283(1-2):49-62. doi: 10.1016/s0378-1119(01)00861-7. Gene. 2002. PMID: 11867212
-
The ADAMTS hyalectanase family: biological insights from diverse species.Biochem J. 2016 Jul 15;473(14):2011-22. doi: 10.1042/BCJ20160148. Biochem J. 2016. PMID: 27407170 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

