Descending interneurons of the stick insect connecting brain neuropiles with the prothoracic ganglion
- PMID: 37651417
- PMCID: PMC10470933
- DOI: 10.1371/journal.pone.0290359
Descending interneurons of the stick insect connecting brain neuropiles with the prothoracic ganglion
Abstract
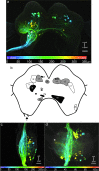
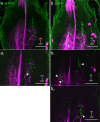
Stick insects respond to visual or tactile stimuli with whole-body turning or directed reach-to-grasp movements. Such sensory-induced turning and reaching behaviour requires interneurons to convey information from sensory neuropils of the head ganglia to motor neuropils of the thoracic ganglia. To date, descending interneurons are largely unknown in stick insects. In particular, it is unclear whether the special role of the front legs in sensory-induced turning and reaching has a neuroanatomical correlate in terms of descending interneuron numbers. Here, we describe the population of descending interneurons with somata in the brain or gnathal ganglion in the stick insect Carausius morosus, providing a first map of soma cluster counts and locations. By comparison of interneuron populations with projections to the pro- and mesothoracic ganglia, we then estimate the fraction of descending interneurons that terminate in the prothoracic ganglion. With regard to short-latency, touch-mediated reach-to-grasp movements, we also locate likely sites of synaptic interactions between antennal proprioceptive afferents to the deutocerebrum and gnathal ganglion with descending or ascending interneuron fibres. To this end, we combine fluorescent dye stainings of thoracic connectives with stainings of antennal hair field sensilla. Backfills of neck connectives revealed up to 410 descending interneuron somata (brain: 205 in 19 clusters; gnathal ganglion: 205). In comparison, backfills of the prothorax-mesothorax connectives stained only up to 173 somata (brain: 83 in 16 clusters; gnathal ganglion: 90), suggesting that up to 60% of all descending interneurons may terminate in the prothoracic ganglion (estimated upper bound). Double stainings of connectives and antennal hair field sensilla revealed that ascending or descending fibres arborise in close proximity of afferent terminals in the deutocerebrum and in the middle part of the gnathal ganglia. We conclude that two cephalothoracic pathways may convey cues about antennal movement and pointing direction to thoracic motor centres via two synapses only.
Copyright: © 2023 Goldammer et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Photohorotaxis Kalmus H., eine neue Reaktionsart, gefunden an den Eilarven von Dixippus morosus. Z vergl Physiol. 1937; 24, pp. 644–655. doi: 10.1007/BF00592302 - DOI
-
- Jander R, Volk-Heinrichs I. Das Strauch-spezifische Perceptor-System der Stabheuschrecke (Carausius morosus). Z vergl Physiol. 1970; 70, pp. 425–447. doi: 10.1007/bf00298197 - DOI
-
- Frantsevich I, Frantsevich L. Space constancy in form perception by the stick insect. Naturwiss. 1996; 83 (7), pp. 323–324. doi: 10.1007/BF01152214 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

