Interventions for fatigue in people with kidney failure requiring dialysis
- PMID: 37651553
- PMCID: PMC10468823
- DOI: 10.1002/14651858.CD013074.pub2
Interventions for fatigue in people with kidney failure requiring dialysis
Abstract
Background: Fatigue is a common and debilitating symptom in people receiving dialysis that is associated with an increased risk of death, cardiovascular disease and depression. Fatigue can also impair quality of life (QoL) and the ability to participate in daily activities. Fatigue has been established by patients, caregivers and health professionals as a core outcome for haemodialysis (HD).
Objectives: We aimed to evaluate the effects of pharmacological and non-pharmacological interventions on fatigue in people with kidney failure receiving dialysis, including HD and peritoneal dialysis (PD), including any setting and frequency of the dialysis treatment.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 18 October 2022 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: Studies evaluating pharmacological and non-pharmacological interventions affecting levels of fatigue or fatigue-related outcomes in people receiving dialysis were included. Studies were eligible if fatigue or fatigue-related outcomes were reported as a primary or secondary outcome. Any mode, frequency, prescription, and duration of therapy were considered.
Data collection and analysis: Three authors independently extracted data and assessed the risk of bias. Treatment estimates were summarised using random effects meta-analysis and expressed as a risk ratio (RR) or mean difference (MD), with a corresponding 95% confidence interval (CI) or standardised MD (SMD) if different scales were used. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results: Ninety-four studies involving 8191 randomised participants were eligible. Pharmacological and non-pharmacological interventions were compared either to placebo or control, or to another pharmacological or non-pharmacological intervention. In the majority of domains, risks of bias in the included studies were unclear or high. In low certainty evidence, when compared to control, exercise may improve fatigue (4 studies, 217 participants (Iowa Fatigue Scale, Modified Fatigue Impact Scale, Piper Fatigue Scale (PFS), or Haemodialysis-Related Fatigue scale score): SMD -1.18, 95% CI -2.04 to -0.31; I2 = 87%) in HD. In low certainty evidence, when compared to placebo or standard care, aromatherapy may improve fatigue (7 studies, 542 participants (Fatigue Severity Scale (FSS), Rhoten Fatigue Scale (RFS), PFS or Brief Fatigue Inventory score): SMD -1.23, 95% CI -1.96 to -0.50; I2 = 93%) in HD. In low certainty evidence, when compared to no intervention, massage may improve fatigue (7 studies, 657 participants (FSS, RFS, PFS or Visual Analogue Scale (VAS) score): SMD -1.06, 95% CI -1.47, -0.65; I2 = 81%) and increase energy (2 studies, 152 participants (VAS score): MD 4.87, 95% CI 1.69 to 8.06, I2 = 59%) in HD. In low certainty evidence, when compared to placebo or control, acupressure may reduce fatigue (6 studies, 459 participants (PFS score, revised PFS, or Fatigue Index): SMD -0.64, 95% CI -1.03 to -0.25; I2 = 75%) in HD. A wide range of heterogenous interventions and fatigue-related outcomes were reported for exercise, aromatherapy, massage and acupressure, preventing our capability to pool and analyse the data. Due to the paucity of studies, the effects of pharmacological and other non-pharmacological interventions on fatigue or fatigue-related outcomes, including non-physiological neutral amino acid, relaxation with or without music therapy, meditation, exercise with nandrolone, nutritional supplementation, cognitive-behavioural therapy, ESAs, frequent HD sections, home blood pressure monitoring, blood flow rate reduction, serotonin reuptake inhibitor, beta-blockers, anabolic steroids, glucose-enriched dialysate, or light therapy, were very uncertain. The effects of pharmacological and non-pharmacological treatments on death, cardiovascular diseases, vascular access, QoL, depression, anxiety, hypertension or diabetes were sparse. No studies assessed tiredness, exhaustion or asthenia. Adverse events were rarely and inconsistently reported.
Authors' conclusions: Exercise, aromatherapy, massage and acupressure may improve fatigue compared to placebo, standard care or no intervention. Pharmacological and other non-pharmacological interventions had uncertain effects on fatigue or fatigue-related outcomes in people receiving dialysis. Future adequately powered, high-quality studies are likely to change the estimated effects of interventions for fatigue and fatigue-related outcomes in people receiving dialysis.
Trial registration: ClinicalTrials.gov NCT02358343 NCT00048035 NCT00155441 NCT00264758 NCT00271999 NCT01721551 NCT00582114 NCT00250536 NCT00618033 NCT01254214 NCT01532297 NCT00255450 NCT02686333 NCT02210377 NCT00093015 NCT00440869 NCT01620580 NCT02361268.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Patrizia Natale: no relevant interests were disclosed
Angela Ju: no relevant interests were disclosed
Giovanni FM Strippoli: no relevant interests were disclosed
Jonathan C Craig: no relevant interests were disclosed
Valeria M Saglimbene: no relevant interests were disclosed
Mark L Unruh: no relevant interests were disclosed
Giovanni Stallone: no relevant interests were disclosed
Allison Jaure: no relevant interests were disclosed
Figures
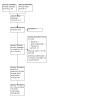
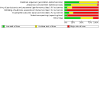
















































































































































































































Update of
- doi: 10.1002/14651858.CD013074
References
References to studies included in this review
Ahmady 2019 {published data only}
-
- Ahmady S, Rezaei M, Khatony A. Comparing effects of aromatherapy with lavender essential oil and orange essential oil on fatigue of hemodialysis patients: A randomized trial. Complementary Therapies in Clinical Practice 2019;36:64-8. [MEDLINE: ] - PubMed
Akizawa 2002 {published data only}
-
- Akizawa T, Koshikawa S, Iida N, Marumo F, Akiba T, Kawaguchi Y, et al. Clinical effects of L-threo-3,4-dihydroxyphenylserine on orthostatic hypotension in hemodialysis patients. Nephron 2002;90(4):384-90. [MEDLINE: ] - PubMed
-
- Akizawa T, Koshikawa S, Iida Y, Marumo F, Kawaguchi Y, Shirai D, et al. Clinical effect of l-threo-3,4-dihydroxyprenylserine (DOPS) on orthostatic hypotension in haemodialysis patients. DOPS Study Group [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A218. [CENTRAL: CN-00261299]
Amini 2016 {published data only}
-
- Amini E, Goudarzi I, Masoudi R, Ahmadi A, Momeni A. Effect of progressive muscle relaxation and aerobic exercise on anxiety, sleep quality, and fatigue in patients with chronic renal failure undergoing hemodialysis. International Journal of Pharmaceutical & Clinical Research 2016;8(12):1634-9. [EMBASE: 614181031]
ASCEND 2016 {published data only}
-
- Cukor D, Rue T, Unruh ML, Heagerty PJ, Cohen SD, Dember LM, et al. Patient treatment adherence in the ASCEND trial for depression in patients undergoing maintenance hemodialysis [abstract no: FR-PO429]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):549. [EMBASE: 633768241]
-
- Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Annals of Internal Medicine 2019;170(6):369-79. [MEDLINE: ] - PubMed
-
- Mehrotra R, Cukor D, Unruh ML, Rue T, Heagerty PJ, Cohen SD, et al. Comparative efficacy of therapies for depression for patients undergoing hemodialysis [abstract no: FR-OR148]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B3.
ASSertID 2015 {published data only}06146268
-
- Chilcot J, Almond MK, Guirguis A, Friedli K, Day C, Davenport A, et al. Self-reported depression symptoms in haemodialysis patients: Bi-factor structures of two common measures and their association with clinical factors. General Hospital Psychiatry 2018;54:31-6. [MEDLINE: ] - PubMed
-
- Chilcot J, Guirguis A, Friedli K, Almond M, Davenport A, Day C, et al. Measuring fatigue using the multidimensional fatigue inventory-20: a questionable factor structure in haemodialysis patients. Nephron 2017;136(2):121-6. [MEDLINE: ] - PubMed
-
- Friedli K, Guirguis A, Almond M, Day C, Chilcot J, Da Silva-Gane M, et al. Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis: a randomized, controlled feasibility trial. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(2):280-6. [MEDLINE: ] - PMC - PubMed
BA16285 2007 {published data only}
-
- Besarab A, Beyer U, Dougherty FC. Long-term intravenous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations in hemodialysis patients [abstract no: W-PO40127]. Nephrology 2005;10(Suppl 1):A312-3. [CENTRAL: CN-00747326]
-
- Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, et al. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19-week, phase II, multicenter, randomized, open-label, dose-finding study with a 12-month extension phase in patients with chronic renal disease. Clinical Therapeutics 2007;29(4):626-39. [MEDLINE: ] - PubMed
-
- Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W-PO40131]. Nephrology 2005;10(Suppl 1):A313-4. [CENTRAL: CN-00602138]
-
- Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W-PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN-00602137]
-
- Dougherty FC, Loghman-Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN-00602143]
Babamohammadi 2006 {published data only}
-
- Babamohammadi H, Khalili H. Effect of a confined program of home-care on the health status of patients recieving hemodialysis. Acta Medica Iranica 2006;44(1):28-32. [EMBASE: 46995829]
Bagheri‐Nesami 2016 {published data only}
-
- Bagheri-Nesami M, Shorofi SA, Nikkhah A, Espahbodi F, Ghaderi Koolaee FS. The effects of aromatherapy with lavender essential oil on fatigue levels in haemodialysis patients: a randomized clinical trial. Complementary Therapies in Clinical Practice 2016 Feb;22:33-7. [MEDLINE: ] - PubMed
Balouchi 2016 {published data only}
-
- Balouchi A, Masinaeinezhad N, Abdallahimohammad A, Firouzkouhi MR, Sepehri Z. Comparison of effects of orange and lavender extract on fatigue in hemodialysis patients. Der Pharmacia Lettre 2016;8(8):50-4. [EMBASE: 610743015]
Barre 1988 {published data only}
-
- Barre PE, Brunelle G, Gascon-Barre M. A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Transactions 1988;34(3):338-41. [MEDLINE: ] - PubMed
Bellinghieri 1983 {published data only}
-
- Bellinghieri G, Savica V, Mallamace A, Di Stefano C, Consolo F, Spagnoli LG, et al. Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients. American Journal of Clinical Nutrition 1983;38(4):523-31. [MEDLINE: ] - PubMed
Bicer 2022 {published data only}
-
- Bicer S, Tasci S. The effect of body acupressure on blood pressure and fatigue levels in individuals suffering from hypotension during hemodialysis: a randomized controlled trial. Alternative Therapies in Health & Medicine 2022;28(2):6-16. [PMID: ] - PubMed
Biniaz 2015 {published data only}
-
- Biniaz V, Tayebi A, Ebadi A, Sadeghi S, Einollahi B. Effect of vitamin C supplementation on marital satisfaction in patients undergoing hemodialysis: a randomized, double-blind and placebo-controlled trial. Saudi Journal of Kidney Diseases & Transplantation 2015;26(3):468-76. [MEDLINE: ] - PubMed
BOLD 2020 {published data only}
-
- Bansal N, Glidden DV, Mehrotra R, Townsend RR, Larson HL, Linke L, et al. A pilot trial targeting home vs. pre-dialysis BP in hemodialysis (HD) patients [abstract no: FR-PO1032]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):710. [EMBASE: 633770522]
Brass 2001 {published data only}
-
- Brass EP, Adler S, Sietsema KE, Hiatt WR, Orlando AM, Amato A, et al. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. American Journal of Kidney Diseases 2001;37(5):1018-28. [MEDLINE: ] - PubMed
Canadian EPO 1990 {published data only}
-
- Anonymous. Effect of recombinant human erythropoietin therapy on blood pressure in hemodialysis patients. Canadian Erythropoietin Study Group. American Journal of Nephrology 1991;11(1):23-6. [MEDLINE: ] - PubMed
-
- Canadian Erythropoietin Study Group. The clinical effects and side-effects of recombinant human erythropoietin (EPO) in anaemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN-00583134]
-
- Canadian Erythropoietin Study Group. The effect of recombinant human erythropoietin (EPO) upon quality of life and exercise capacity of anemic patients on chronic hemodialysis [abstract]. Kidney International 1990;37(1):278. [CENTRAL: CN-00583135]
-
- Donnelly S, Posen G, Ali M. Oral iron absorption in hemodialysis (HD), patients treated with erythropoietin (EPO) [abstract]. Kidney International 1990;37(1):293. [CENTRAL: CN-00747347]
Cecen 2021 {published data only}
-
- Cecen S, Lafci D. The effect of hand and foot massage on fatigue in hemodialysis patients: a randomized controlled trial. Complementary Therapies in Clinical Practice 2021;43:101344. [MEDLINE: ] - PubMed
Chang 2010 {published data only}
-
- Chang Y, Cheng SY, Lin M, Gau FY, Chao YF. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. International Journal of Nursing Studies 2010;47(11):1383-8. [MEDLINE: ] - PubMed
Chen 2008a {published data only}
-
- Chen HY, Chiang CK, Wang HH, Hung KY, Lee YJ, Peng YS, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. American Journal of Kidney Diseases 2008;52(2):314-23. [MEDLINE: ] - PubMed
Chen 2011a {published data only}
-
- Chen HY, Cheng IC, Pan YJ, Chiu YL, Hsu SP, Pai MF, et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney International 2011;80(4):415-22. [MEDLINE: ] - PubMed
Cho 2004 {published data only}
-
- Cho YC, Tsay SL. The effect of acupressure with massage on fatigue and depression in patients with end-stage renal disease. Journal of Nursing Research 2004;12(1):51-9. [MEDLINE: ] - PubMed
Chow 2010 {published data only}
-
- Chow SK, Wong FK. Health-related quality of life in patients undergoing peritoneal dialysis: effects of a nurse-led case management programme. Journal of Advanced Nursing 2010;66(8):1780-92. [MEDLINE: ] - PubMed
-
- Wong FK, Chow SK, Chan TM. Evaluation of a nurse-led disease management programme for chronic kidney disease: a randomized controlled trial. International Journal of Nursing Studies 2010;47(3):268-78. [MEDLINE: ] - PubMed
Dashti‐Khavidaki 2013 {published data only}
-
- Dashti-Khavidaki S, Sharif Z, Khalili H, Badri S, Alimadadi A, Ahmadi F, et al. The use of pharmaceutical care to improve health-related quality of life in hemodialysis patients in Iran. International Journal of Clinical Pharmacy 2013;35(2):260-7. [MEDLINE: ] - PubMed
Duggal 2019 {published data only}
-
- Duggal V, Abra GE, Reiterman M, Hussein WF, Schiller B. Preliminary analysis of the effect of blood flow rate reduction on post-dialysis fatigue [abstract no: TH-PO313]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):194-5. [EMBASE: 633737000]
-
- Duggal V, Hussein WF, Reiterman M, Sun SJ, Abra GE, Schiller B. The effect of blood flow rate on dialysis recovery time in patients undergoing maintenance hemodialysis: a prospective, parallel-group, randomized controlled trial. Hemodialysis International 2019;23(2):223-9. [MEDLINE: ] - PubMed
Eroglu 2022 {published data only}
-
- Eroglu H, Gok Metin Z. Benson relaxation technique combined with music therapy for fatigue, anxiety, and depression in hemodialysis patients: a randomized controlled trial. Holistic Nursing Practice 2022;36(3):139-48. [PMID: ] - PubMed
Fatigue‐HD 2019 {published data only}
-
- Farragher J, Davis J, Polatajko H, Elliott M, Ravani P, Manns B, et al. Exploring the life participation experiences of people on chronic hemodialysis who participated in an energy management education program [abstract no: POS-572]. Kidney International Reports 2021;6(4 Suppl):S252. [EMBASE: 2011684286]
-
- Farragher JF, Ravani P, Manns BJ, Elliott MJ, Thomas CM, Donald M, et al. A pilot randomized control trial of an energy management program for adults on chronic hemodialysis with fatigue: The FATIGUE-HD study [abstract no: PO1077]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):366. [EMBASE: 633704070]
Fatouros 2010 {published data only}
-
- Fatouros IG, Douroudos I, Panagoutsos S, Pasadakis P, Nikolaidis MG, Chatzinikolaou A, et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Medicine & Science in Sports & Exercise 2010;42(10):1809-18. [MEDLINE: ] - PubMed
-
- Frequent Hemodialysis Network (FHN) Trial Group. The Frequent Hemodialysis Network multicenter randomized trial of in-center daily hemodialysis [abstract no: F-PO009]. Journal of the American Society of Nephrology 2006;17(Abstracts):338A.
FHN DAILY 2007 {published data only}
-
- Beck GJ, Chertow GM, Eggers PW, Greene T, Larive B, Levin NW, et al. Influence of methodology on left ventricular mass measurement by cardiac magnetic resonance in the Frequent Hemodialysis Network (FHN) nocturnal trial [abstract no: SA-PO437]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):726A.
-
- Chan CT, Beck GJ, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, et al. Effects on ventricular volumes by frequent hemodialysis: results from the Frequent Hemodialysis Network (FHN) trials [abstract no: SA-OR391]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):94A.
-
- Chan CT, Chertow GM, Daugirdas JT, Greene T, Kotanko P, Larive B, et al. Effects of daily hemodialysis on heart rate variability: results from the Frequent Hemodialysis Network (FHN) Daily trial [abstract no: SA-OR038]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):74A. - PMC - PubMed
FHN NOCTURNAL 2007 {published data only}
-
- Beck GJ, Chertow GM, Eggers PW, Greene T, Larive B, Levin NW, et al. Influence of methodology on left ventricular mass measurement by cardiac magnetic resonance in the Frequent Hemodialysis Network (FHN) nocturnal trial [abstract no: SA-PO437]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):726A.
-
- Chan CT, Beck GJ, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, et al. Effects on ventricular volumes by frequent hemodialysis: results from the Frequent Hemodialysis Network (FHN) trials [abstract no: SA-OR391]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):94A.
-
- Chan CT, Kaysen GA, Beck GJ, Rocco MV, Kliger AS. The effect of frequent hemodialysis on phosphate and fibroblast growth factor 23: results from the Frequent Hemodialysis Network trials [abstract no: TH-PO347]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):204.
Figueiredo 2018 {published data only}
-
- Figueiredo PH, Lima MM, Costa HS, Martins JB, Flecha OD, Goncalves PF, et al. Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: a randomized clinical trial. PLoS ONE [Electronic Resource] 2018;13(7):e0200727. [MEDLINE: ] - PMC - PubMed
Foley 2000 {published data only}
-
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. A randomized controlled trial of complete vs partial correction of anemia in hemodialysis patients with asymptomatic concentric lV hypertrophy or lV dilation [abstract no: A1064]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):208A.
-
- Foley RN, Parfrey PS, Morgan J, Barre P, Campbell P, Cartier P, et al. Diastolic dysfunction in hemodialysis patients: the Canadian Normalization of Hemoglobin Study Group [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):261A. [CENTRAL: CN-00550674]
-
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney International 2000;58(3):1325-35. [MEDLINE: ] - PubMed
-
- Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, et al. Hemoglobin levels and hospitalization in hemodialysis patients without symptomatic cardiac disease [abstract no: SA-PO818]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):432A. [CENTRAL: CN-00445362]
-
- Wells GA, Coyne D, Lee KM, Foley RN, Parfrey PS, et al. Quality of life effects of normalization of hemoglobin in asymptomatic hemodialysis patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):230A. [CENTRAL: CN-00448336]
Fukuda 2015 {published data only}
-
- Fukuda S, Koyama H, Fujii H, Hirayama Y, Tabata T, Okamura M, et al. Effect of nutritional support on reactivation of fatigue-related human herpers virus 6/7 reactivation in hemodialysis patients: a randomized double-blind placebo-controlled study [abstract no: F-PO1486]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):453A.
-
- Fukuda S, Koyama H, Kondo K, Fujii H, Hirayama Y, Tabata T, et al. Effects of nutritional supplementation on fatigue, and autonomic and immune dysfunction in patients with end-stage renal disease: a randomized, double-blind, placebo-controlled, multicenter trial. PLoS ONE [Electronic Resource] 2015;10(3):e0119578. [MEDLINE: ] - PMC - PubMed
-
- Koyama H, Fukuda S, Fujii H, Hirayama Y, Tabata T, Okamura M, et al. Effects of nutritional support on fatigue and autonomic dysfunction in patients with end-stage renal diseases: a randomized double-blind placebo-controlled study [abstract no: F-PO1511]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):458A.
Grigoriou 2021 {published data only}
-
- Grigoriou SS, Giannaki CD, George K, Karatzaferi C, Zigoulis P, Eleftheriadis T, et al. A single bout of hybrid intradialytic exercise did not affect left-ventricular function in exercise-naive dialysis patients: a randomized, cross-over trial. International Urology & Nephrology 2021;53(1):771–84. [MEDLINE: ] - PubMed
Habibzadeh 2020 {published data only}
Hadadian 2016 {published data only}
Hadadian 2018 {published data only}
-
- Hadadian F, Jalalvandi F, Karimi S, Abdi A, Salari N, Ghobadi A. Studying the effect of progressive muscle relaxation technique on fatigue in hemodialysis patients-Kermanshah-Iran. Annals of Tropical Medicine & Public Health 2018;11(1):8-12. [EMBASE: 630312180]
Hasankhani 2013 {published data only}
-
- Hasankhani H, Ghaderi F, Lakdizaji S, Nahamin M. The effect of the slow-stroke back massage on fatigue of dialyzed patients. International Research Journal of Applied & Basic Science 2013;4(10):3004-8.
Hassanzadeh 2018 {published data only}
-
- Hassanzadeh M, Kiani F, Bouya S, Zarei M. Comparing the effects of relaxation technique and inhalation aromatherapy on fatigue in patients undergoing hemodialysis. Complementary Therapies in Clinical Practice 2018;31:210-4. [MEDLINE: ] - PubMed
HDPAL 2014 {published data only}
-
- Georgianos PI, Agarwal R. Aortic stiffness, ambulatory blood pressure, and predictors of response to antihypertensive therapy in hemodialysis. American Journal of Kidney Diseases 2015;66(2):305-12. [MEDLINE: ] - PubMed
Huang 2021 {published data only}
-
- Huang HY, Hung KS, Yeh ML, Chou HL, Yeh AL, Liao TY. Breathing-based leg exercises during hemodialysis improve quality of life: a randomized controlled trial. Clinical Rehabilitation 2020;35(8):1175-84. [MEDLINE: ] - PubMed
Jalalian 2015 {published data only}
-
- Jalalian Z, Varayi S, Nejad MS. Effects of aromatherapy on fatigue and quality of life in patients undergoing hemodialysis [abstract]. Avicenna Journal of Phytomedicine 2015;5(Suppl 1):66-7. [EMBASE: 72156754]
Johansen 1999 {published data only}
-
- Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients on dialysis: a randomized, placebo-controlled trial [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):212A. [CENTRAL: CN-00445936]
-
- Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA 1999;281(14):1275-81. [MEDLINE: ] - PubMed
Johansen 2006 {published data only}
-
- Johansen KL, Painter PL, Gordon P, Doyle J, Sakkas GK. Effects of resistance exercise training and anabolic steroid treatment among hemodialysis patients: results of the NEXT study [abstract no: SU-PO382]. Journal of the American Society of Nephrology 2004;15(Oct):617A. [CENTRAL: CN-00550563]
-
- Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. Journal of the American Society of Nephrology 2006;17(8):2307-14. [MEDLINE: ] - PubMed
Kaplin Serin 2020 {published data only}
-
- Kaplan Serin E, Ovayolu N, Ovayolu O. The effect of progressive relaxation exercises on pain, fatigue, and quality of life in dialysis patients. Holistic Nursing Practice 2020;34(2):121-8. [MEDLINE: ] - PubMed
Karadag 2019 {published data only}
-
- Karadag E, Samancioglu Baglama S. The effect of aromatherapy on fatigue and anxiety in patients undergoing hemodialysis treatment: a randomized controlled study. Holistic Nursing Practice 2019;33(4):222-9. [MEDLINE: ] - PubMed
Konstadinidou‐ND 2002 {published data only}
-
- Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. Journal of Rehabilitation Medicine 2002;34(1):40-5. [MEDLINE: ] - PubMed
Krase 2022 {published data only}
-
- Krase AA, Terzis G, Giannaki CD, Stasinaki AN, Wilkinson TJ, Smith AC, et al. Seven months of aerobic intradialytic exercise training can prevent muscle loss in haemodialysis patients: an ultrasonography study. International Urology & Nephrology 2022;54(2):447-56. [PMID: ] - PubMed
Lazarus 2020 {published data only}
-
- Lazarus ER, Deva Amirtharaj A, Jacob D, Chandrababu R, Isac C. The effects of an olive-oil massage on hemodialysis patients suffering from fatigue at a hemodialysis unit in southern India - a randomized controlled trial. Journal of Complementary & Integrative Medicine 2020;18(2):397-403. [MEDLINE: ] - PubMed
Leski 1979 {published data only}
-
- Leski M, Niethammer T, Wyss T. Glucose-enriched dialysate and tolerance to maintenance hemodialysis. Nephron 1979;24(6):271-3. [MEDLINE: ] - PubMed
Li 2014b {published data only}
-
- Li J, Wang H, Xie H, Mei G, Cai W, Ye J, et al. Effects of post-discharge nurse-led telephone supportive care for patients with chronic kidney disease undergoing peritoneal dialysis in China: a randomized controlled trial. Peritoneal Dialysis International 2014;34(3):278-88. [MEDLINE: ] - PMC - PubMed
Lillevang 1990 {published data only}
-
- Lillevang ST, Pedersen FB. Quality of life of hemodialysis patients before and after erythropoietin therapy. A double-blind, randomized, placebo controlled study [Haemodialysepatienters livskvalitet for og efter erytropoietinbehandling. En dobbeltblind, randomiseret, placebokontrolleret undersogelse]. Ugeskrift for Laeger 1990;152(41):2999-3002. [MEDLINE: ] - PubMed
Lin 2011 {published data only}
-
- Lin CH, Lee LS, Su LH, Huang TC, Liu CF. Thermal therapy in dialysis patients - a randomized trial. American Journal of Chinese Medicine 2011;39(5):839-51. [MEDLINE: ] - PubMed
Linde 2001 {published data only}
-
- Danielson BG, Furuland H, Ahlmen J, Christensson A, Linde T, Strombom U. Scandinavian study of normalizing hemoglobin with Rhu-EPO in end stage renal failure [abstract no: A0822]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):160A. [CENTRAL: CN-00550642]
-
- Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrology Dialysis Transplantation 2003;18(2):353-61. [MEDLINE: ] - PubMed
-
- Furuland H, Linde T, Danielson BG. Cardiac function in patients with end-stage renal disease after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN-00445402]
-
- Furuland H, Linde T, Danielson BG. Dialysis adequacy after normalization of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):296A. [CENTRAL: CN-00445403]
-
- Furuland H, Linde T, Danielson BG. Physical exercise capacity in patients with end-stage renal disease after normalizaton of hemoglobin with erythropoietin (EPO) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):337A. [CENTRAL: CN-00445404]
Mohajeranirad 2021 {published data only}
-
- Mohajeranirad M, Saeidi N, Kamali Nejad M, Almasi-Hashiani A, Salehi M, Latifi SA. Effects of Helichrysum psudoplicatum supplementation on pruritus intensity, fatigue, quality of life and anorexia in hemodialysis patients: a randomized, double-blind placebo-controlled trial. Hormone Molecular Biology & Clinical Investigation 2021;43(2):211-8. [PMID: ] - PubMed
Mohamed 2013 {published data only}
-
- Mohamed A, Alam JH, Barre P, Vasilevsky M, Beauchemin R, Iqbal S. A randomized controlled trial to examine the effect of dialysate glucose concentration on quality of life among hemodialysis patients with type 2 diabetes mellitus [abstract]. Hemodialysis International 2013;17(1):167. [EMBASE: 71022215]
Mohamed 2014 {published data only}
-
- Mohamed SA. The effectiveness of an educational intervention on fatigue in hemodialysis patients: a randomized controlled trial. Journal of Nursing & Health Science 2014;3(4):40-50. [CENTRAL: CN-01657905]
Mohammadpourhodki 2021 {published data only}
-
- Mohammadpourhodki R, Sadeghnezhad H, Ebrahimi H, Basirinezhad MH, Maleki M, Bossola M. The effect of aromatherapy massage with lavender and citrus aurantium essential oil on quality of life of patients on chronic hemodialysis: a parallel randomized clinical trial study. Journal of Pain & Symptom Management 2021;61(3):456-63.e1. [MEDLINE: ] - PubMed
Motedayen 2014 {published data only}
Muz 2017 {published data only}
-
- Muz G, Tasci S. Effect of aromatherapy via inhalation on the sleep quality and fatigue level in people undergoing hemodialysis. Applied Nursing Research 2017;37:28-35. [MEDLINE: ] - PubMed
Ozdemir 2013 {published data only}
-
- Ozdemir G, Ovayolu N, Ovayolu O. The effect of reflexology applied on haemodialysis patients with fatigue, pain and cramps. International Journal of Nursing Practice 2013;19(3):265-73. [MEDLINE: ] - PubMed
Parfrey 2005 {published data only}
-
- Foley RN, Parfrey PS, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D, et al. The effect of higher haemoglobin levels on left ventricular cavity volume in patients starting haemodialysis: a blinded, randomised, controlled trial in 596 patients without symptomatic cardiac disease [abstract]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:217.
PEDAL 2020 {published data only}
-
- Greenwood SA, Koufaki P, Macdonald J, Bhandari S, Burton J, Dasgupta I, et al. The PrEscription of intraDialytic exercise to improve quAlity of Life in patients with chronic kidney disease trial: study design and baseline data for a multicentre randomized controlled trial. Clinical Kidney Journal 2021;14(5):1345–55. [MEDLINE: ] - PMC - PubMed
Pellizzaro 2013 {published data only}
-
- Pellizzaro CO, Thome FS, Veronese FV. Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Renal Failure 2013;35(2):189-97. [MEDLINE: ] - PubMed
Picariello 2018 {published data only}91238019
-
- Picariello F, Moss-Morris R, Macdougall IC, Norton S, Da Silva-Gane M, Farrington K, et al. Cognitive-behavioural therapy (CBT) for renal fatigue (BReF): a feasibility randomised-controlled trial of CBT for the management of fatigue in haemodialysis (HD) patients. BMJ Open 2018;8(3):e020842. [MEDLINE: ] - PMC - PubMed
-
- Picariello F, Moss-Morris R, Norton S, Macdougall IC, Da Silva-Gane M, Farrington K, et al. Feasibility trial of cognitive behavioral therapy for fatigue in hemodialysis (BReF Intervention). Journal of Pain & Symptom Management 2021;61(6):1234-46.e5. [MEDLINE: ] - PubMed
Raimann 2010 {published data only}
-
- Ferrario M, Raimann JG, Thijssen S, Signorini MG, Kruse A, Diaz-Buxo JA, et al. Effects of dialysate glucose concentration on heart rate variability in chronic hemodialysis patients: Results of a prospective randomized trial. Kidney & Blood Pressure Research 2011;34(5):334-43. [EMBASE: 21613795] - PubMed
-
- Ferrario M, Signorini MG, Raimann J, Kruse A, Thijssen S, Kotanko P, et al. The effects of dialysate dextrose concentration of the autonomic control in diabetic and non-diabetic populations [abstract no: M541]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
-
- Kruse A, Raimann J, Kuntsevich V, Dabel P, Arthur B, Thijssen S, et al. Arrhythmias in hemodialysis patients: a comparison between 100 and 200 mg/dl dialysate dextrose concentration [abstract no: SU606]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
-
- Raimann J, Kruse A, Kuntsevich V, Dabel P, Thijssen S, Kotanko P, et al. Prospective randomized study of two levels of dialysate dextrose concentration: 100 versus 200 mg/dL [abstract no: SU603]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
-
- Raimann J, Kruse A, Kuntsevich V, Winchester J, Diaz-Buxo J, Kotanko P, et al. Fatigue in chronic hemodialysis patients -results from a prospective randomized cross-over trial of 200 mg/dL and 100 mg/dL dialysate dextrose concentrations [abstract no: TH-PO339]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):190A.
Reilly‐Spong 2015 {published data only}
-
- Gross CR, Reilly-Spong M, Park T, Zhao R, Gurvich O. Randomized clinical trial of telephone-adapted mindfulness training on anxiety, symptom distress, and quality of life in patients with progressive renal disease [abstract]. Journal of Alternative & Complementary Medicine 2016;22(6):A77. [EMBASE: 611808428]
Roshanravan 2016 {published data only}
-
- Roshanravan M, Jouybari L, Bahrami-Taghanaki H, Vakili MA, Sanagoo A, Amini Z. Effect of foot reflexology on fatigue in patients undergoing hemodialysis: a sham-controlled randomized trial. Journal of Mazandaran University of Medical Sciences 2016;26(137):32-41. [EMBASE: 610668018]
Sabouhi 2013 {published data only}
Sajadi 2016 {published data only}
-
- Sajadi M, Gholami Z, Hekmatpour D, Soltani P, Haghverdi F. Cold dialysis solution for hemodialysis patients with fatigue: a cross-over study [Erratum in: Iran J Kidney Dis. 2016 Nov;10 (6):419 PMID: 27904003]. Iranian Journal of Kidney Diseases 2016;10(5):319-24. [PMID: ] - PubMed
Salehi 2020 {published data only}
Sang 1997 {published data only}
-
- Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. American Journal of Kidney Diseases 1997;29(5):669-77. [MEDLINE: ] - PubMed
Schardong 2021 {published data only}
-
- Schardong J, Falster M, Sisto IR, Barbosa AP, Normann TC, Souza KS, et al. Photobiomodulation therapy increases functional capacity of patients with chronic kidney failure: randomized controlled trial. Lasers in Medical Science 2021;36(1):119-29. [MEDLINE: ] - PubMed
Schmitz 2016 {published data only}
-
- Schmitz M, Loke O, Fach B, Kalb K, Heering PJ, Meinke D, et al. Effects of citrate dialysate in chronic dialysis: a multicentre randomized crossover study. Nephrology Dialysis Transplantation 2016;31(8):1327-34. [MEDLINE: ] - PubMed
Semeniuk 2000 {published data only}
-
- Semeniuk J, Shalansky KF, Taylor N, Jastrzebski J, Cameron EC. Evaluation of the effect of intravenous l-carnitine on quality of life in chronic hemodialysis patients. Clinical Nephrology 2000;54(6):470-7. [MEDLINE: ] - PubMed
Shahdadi 2016 {published data only}
-
- Shahdadi H, Hodki RM, Abadi AA, Sheikh A, Moghadasi A. The effect of slow stroke back massage on fatigue in patients undergoing hemodialysis: a randomized clinical trial. International Journal of Pharmacy & Technology 2016;8(3):16016-23. [EMBASE: 612952307]
Singer 2010 {published data only}
-
- Singer R. Vitamin C supplementation in kidney failure: effect on uraemic symptoms [abstract no: 066]. Nephrology 2010;15(Suppl 4):44. [CENTRAL: 70467070] - PubMed
-
- Singer RF. Vitamin C supplementation in kidney failure: effect on uraemic symptoms. Nephrology Dialysis Transplantation 2011;26(2):614-20. [MEDLINE: ] - PubMed
Singh 2003 {published data only}
-
- Singh NP, Banal R, Thakur A, Kohli R, Bansal RC, Agarwal SK. Effect of membrane composition on cytokine production and clinical symptoms during hemodialysis: a crossover study. Renal Failure 2003;25(3):419-30. [MEDLINE: ] - PubMed
Singh 2008a {published data only}
-
- Singh A, Hertel J, Bernardo M, Baptista J, Kausz A, Brenner L, et al. A double-blind, placebo-controlled, randomized phase III study of the safety of ferumoxytol as a new intravenous iron replacement therapy [abstract no: 29]. American Journal of Kidney Diseases 2007;49(4):A32. [CENTRAL: CN-00716121]
-
- Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. American Journal of Kidney Diseases 2008;52(5):907-15. [MEDLINE: ] - PubMed
Sklar 1998 {published data only}
-
- Sklar AH, Beezhold DH, Dreisbach AW, Hendrickson T, Riesenberg LA. Effect of a biocompatible membrane on cytokines and postdialysis fatigue [abstract no: P1343]. Nephrology 1997;3(Suppl 1):S408.
-
- Sklar AH, Beezhold DH, Newman N, Hendrickson T, Dreisbach AW. Postdialysis fatigue: lack of effect of a biocompatible membrane. American Journal of Kidney Diseases 1998;31(6):1007-10. [MEDLINE: ] - PubMed
Sklar 1999 {published data only}
-
- Sklar A, Newman N, Scott R, Semenyuk L, Schultz J, Fiacco V. Identification of factors responsible for postdialysis fatigue. American Journal of Kidney Diseases 1999;34(3):464-70. [MEDLINE: ] - PubMed
SOCIABLE 2017 {published data only}
-
- Crews DC, Delaney AM, Walker JL, Cudjoe TK, Evelyn-Gustave A, Roth J, et al. Improving physical function and social networks of older adults with ESRD: development and testing of seniors optimizing community integration to advance better living with ESRD (SOCIABLE) [abstract no: FR-PO924]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):643. [EMBASE: 633700279]
-
- Crews DC, Delaney AM, Walker Taylor JL, Cudjoe TK, Nkimbeng M, Roberts L, et al. Pilot intervention addressing social support and functioning of low socioeconomic status older adults with ESRD: The Seniors Optimizing Community Integration to Advance Better Living with ESRD (SOCIABLE) Study. Kidney Medicine 2019;1(1):13-20. [PMID: ] - PMC - PubMed
Soliman 2015 {published data only}
-
- Soliman HM. Effect of intradialytic exercise on fatigue, electrolytes level and blood pressure in hemodialysis patients: a randomized controlled trial. Journal of Nursing Education & Practice 2015;5(11):16-28.
Su 2009 {published data only}
-
- Su LH, Wu KD, Lee LS, Wang H, Liu CF. Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. American Journal of Chinese Medicine 2009;37(2):215-26. [MEDLINE: ] - PubMed
Suzuki 2018 {published data only}
-
- Morton RL. The symptom monitoring with feedback trial (swift): A novel registry-based cluster randomised controlled trial among Australian and New Zealand adults with end-stage kidney disease managed on haemodialysis [abstract]. Nephrology 2018;23(Suppl 3):82-3. [EMBASE: 623841124]
-
- Suzuki T, Ikeda M, Minami M, Matayoshi Y, Nakao M, Nakamura T, et al. Beneficial effect of intradialytic electrical muscle stimulation in hemodialysis patients: a randomized controlled trial. Artificial Organs 2018;42(9):899-910. [MEDLINE: ] - PubMed
SWIFT 2020 {published data only}
-
- Duncanson E, Bennett PN, Viecelli A, Dansie K, Handke W, Tong A, et al. Feasibility and acceptability of e-PROMs data capture and feedback among patients receiving haemodialysis in the Symptom monitoring WIth Feedback Trial (SWIFT) pilot: protocol for a qualitative study in Australia. BMJ Open 2020;10(11):e039014. [MEDLINE: ] - PMC - PubMed
-
- Morton R. Study protocol for the symptom monitoring with feedback trial (SWIFT): a novel registry-based cluster randomised trial among adults with end-stage kidney disease managed on haemodialysis [abstract no: SAT-037]. Kidney International Reports 2019;4(7 Suppl):S19. [EMBASE: 2002179660]
-
- Morton RL, Dansie K, Bennett PN, Duncanson E, Viecelli AK, Jesudason S, et al. Feasibility and acceptability of symptom monitoring with feedback trial (SWIFT) for adults on hemodialysis: a pilot ANZDATA registry-based cluster randomized trial [abstract no: PO1080]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):367. [EMBASE: 633704095]
-
- Viecelli A, Dansie K, McDonald S, Jesudason S, Duncanson E, Bennett P, et al. Symptom monitoring with feedback trial (SWIFT) pilot to explore the feasibility and acceptability of electronic patient reported outcome measures (e-proms) data capture and feedback [abstract no: MO037]. Nephrology Dialysis Transplantation 2020;35(Suppl 3):iii137. [EMBASE: 633422179]
Thomas 2017 {published data only}
-
- Thomas Z, Novak M, Platas SG, Gautier M, Holgin AP, Fox R, et al. Brief mindfulness meditation for depression and anxiety symptoms in patients undergoing hemodialysis: a pilot feasibility study. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(12):2008-15. [MEDLINE: ] - PMC - PubMed
Tsai 2016 {published data only}
-
- Tsai MY, Wu CH, Huang YC, Chen SY, Ng HY, Su YJ, et al. Treatment of intradialytic hypotension with an herbal acupoint therapy in hemodialysis patients: a randomized pilot study. Complementary Therapies in Medicine 2018;38:67-73. [MEDLINE: ] - PubMed
Tsay 2004a {published data only}
-
- Tsay SL. Acupressure and fatigue in patients with end-stage renal disease-a randomized controlled trial. International Journal of Nursing Studies 2004;41(1):99-106. [MEDLINE: ] - PubMed
Tsay 2004b {published data only}
-
- Tsay SL, Cho YC, Chen ML. Acupressure and transcutaneous electrical acupoint stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. American Journal of Chinese Medicine 2004;32(3):407-16. [MEDLINE: ] - PubMed
Unal 2016 {published data only}
-
- Unal KS, Balci Akpinar R. The effect of foot reflexology and back massage on hemodialysis patients' fatigue and sleep quality. Complementary Therapies in Clinical Practice 2016;24:139-44. [MEDLINE: ] - PubMed
Varaei 2020 {published data only}
-
- Varaei S, Jalalian Z, Yekani Nejad MS, Shamsizadeh M. Comparison the effects of inhalation and massage aromatherapy with lavender and sweet orange on fatigue in hemodialysis patients: a randomized clinical trial. Journal of Complementary & Integrative Medicine 2020;18(1):193-200. [MEDLINE: ] - PubMed
VENOUS 2020 {published data only}
-
- Masakne I. Verification of nutrition maintaining effects by anti-thrombotic PMMA membrane (VENUS Study) [abstract no: SAT-256]. Kidney International Reports 2020;5(3 Suppl):S108-9. [EMBASE: 2005255703]
Vishnevskii 2014 {published data only}
-
- Vishnevskii KA, Rumyantsev AS, Zemchenkov AY, Smirnov AV. Improvement of the physical ability and hemodialysis efficiency due to transcutaneous electrical muscle stimulation of lower extremities [abstract no: SP506]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii240-1. [EMBASE: 71492133]
Yurtkuran 2007 {published data only}
-
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complementary Therapies in Medicine 2007;15(3):164-71. [MEDLINE: ] - PubMed
References to studies excluded from this review
CHAIR 2015 {published data only}
-
- Matsufuji S, Shoji T, Yano Y, Tsujimoto Y, Kishimoto H, Tabata T, et al. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. Journal of Renal Nutrition 2015;25(1):17-24. [MEDLINE: ] - PubMed
Churchill 1987 {published data only}
-
- Churchill DN, Taylor DW, Sackett DL, Shimizu AG. Reuse of dialyzers: a multiple cross-over trial with random allocation to treatment order [abstract]. Kidney International 1987;31(1):230. [CENTRAL: CN-00550365]
-
- Churchill DN, Taylor DW, Shimizu AG, Beecroft ML, Singer J, Barnes CC, et al. Dialyzer re-use--a multiple crossover study with random allocation to order of treatment. Nephron 1988;50(4):325-31. [MEDLINE: ] - PubMed
Dashti‐Khavidaki 2011 {published data only}
-
- Dashti-Khavidaki S, Chamani N, Khalili H, Hajhossein TA, Ahmadi F, Lessan-Pezeshki M, et al. Comparing effects of clonazepam and zolpidem on sleep quality of patients on maintenance hemodialysis. Iranian journal of Kidney Diseases 2011;5(6):404-9. [MEDLINE: ] - PubMed
Eglence 2013 {published data only}
-
- Eglence R, Karatas N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Alternative Therapies in Health & Medicine 2013;19(6):23-31. [MEDLINE: ] - PubMed
Gram 1998 {published data only}
-
- Gram J, Hansen TB, Jensen PB, Christensen JH, Ladefoged S, Pedersen FB. The effect of recombinant human growth hormone treatment on bone and mineral metabolism in haemodialysis patients. Nephrology Dialysis Transplantation 1998;13(6):1529-34. [MEDLINE: ] - PubMed
-
- Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, et al. Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clinical Nephrology 2000;53(2):99-107. [MEDLINE: ] - PubMed
-
- Jensen PB, Ekelund B, Nielsen FT, Baumbach L, Pedersen FB, Oxhoj H. Changes in cardiac muscle mass and function in hemodialysis patients during growth hormone treatment. Clinical Nephrology 2000;53(1):25-32. [MEDLINE: ] - PubMed
-
- Jensen PB, Hansen TB, Frystyk J, Ladefoged SD, Pedersen FB, Christiansen JS. Growth hormone, insulin-like growth factors and their binding proteins in adult hemodialysis patients treated with recombinant human growth hormone. Clinical Nephrology 1999;52(2):103-9. [MEDLINE: ] - PubMed
-
- Jensen PB, Hansen TB, Oxhoj H, Froberg K, Ekelund B, Nielsen FT, et al. What are the clinical benefits of correcting the catabolic state in haemodialysis patients? British Journal of Clinical Practice Supplement 1996;85:47-51. [MEDLINE: ] - PubMed
Heshmatifar 2015 {published data only}
-
- Heshmatifar N, Sadeghi H, Mahdavi A, Shegarf Nakhaie MR, Rakhshani MH. The effect of Benson relaxation technique on depression in patients undergoing hemodialysis. Journal of Babol University of Medical Sciences 2015;17(8):34-40. [EMBASE: 605531696]
Heshmati Far 2015 {published data only}
-
- Heshmati Far N, Salari M, Rakhshani MH, Borzoee F, Sahebka M. The effects of Benson relaxation technique on activities of daily living in hemodialysis patients; a single-blind, randomized, parallel-group, controlled trial study. Complementary Therapies in Clinical Practice 2020;39:101133. [MEDLINE: ] - PubMed
Laupacis 1992 {published data only}
-
- Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on haemodialysis [Erratum in: Nephron 1992;61(2):248]. Nephron 1992;60(3):302-6. [MEDLINE: ] - PubMed
Macagnan 2019 {published data only}
-
- Macagnan FE, Baroni BM, Cristofoli EZ, Godoy M, Schardong J, Plentz RD. Acute effect of photobiomodulation therapy on handgrip strength of chronic kidney disease patients during hemodialysis. Lasers in Medical Science 2019;34(4):835-40. [MEDLINE: ] - PubMed
Nakamoto 2008 {published data only}
-
- Nakamoto H, Mimura T, Honda N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodialysis International 2008;12 Suppl 2:S9-14. [MEDLINE: ] - PubMed
Sharp 2005 {published data only}
-
- Sharp J, Wild MR, Gumley AI, Deighan CJ. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trial. American Journal of Kidney Diseases 2005;45(6):1046-57. [MEDLINE: ] - PubMed
Shimizu 1983 {published data only}
-
- Shimizu AG, Taylor DW, Sackett DL, Smith EK, Barnes CC, Hoda P, et al. Reducing patient morbidity from high-efficiency hemodialysis: a double-blind crossover trial. Transactions - American Society for Artificial Internal Organs 1983;29:666-8. [MEDLINE: ] - PubMed
Siami 1991 {published data only}
-
- Siami G, Clinton ME, Mrak R, Griffis J, Stone W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991;57(3):306-13. [MEDLINE: ] - PubMed
Tawney 2000 {published data only}
-
- Tawney KW, Tawney PJ, Hladik G, Hogan SL, Falk RJ, Weaver C, et al. The life readiness program: a physical rehabilitation program for patients on hemodialysis. American Journal of Kidney Diseases 2000;36(3):581-91. [MEDLINE: ] - PubMed
-
- Tawney KW, Tawney PJ, Hladik GA, Hogan SL, Moore DT, Falk RJ. The life readiness program: a rehabilitation program for end-stage renal disease patients on hemodialysis [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):270A. [CENTRAL: CN-00583258] - PubMed
TREAT 2005 {published data only}
-
- Charytan DM, Solomon SD, Ivanovich P, Remuzzi G, Cooper ME, McGill JB, et al. ESRD after heart failure, myocardial infarction, or stroke in type 2 diabetic patients with CKD. American Journal of Kidney Diseases 2017;70(4):522-31. [MEDLINE: ] - PubMed
-
- Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. American Journal of Kidney Diseases 2011;58(5):717-28. [MEDLINE: ] - PubMed
-
- Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJ, Solomon SD, et al. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. American Heart Journal 2015;170(2):322-9. [MEDLINE: ] - PubMed
Tsai 2015 {published data only}
-
- Tsai SH, Wang MY, Miao NF, Chian PC, Chen TH, Tsai PS. CE: original research: The efficacy of a nurse-led breathing training program in reducing depressive symptoms in patients on hemodialysis: a randomized controlled trial. American Journal of Nursing 2015;115(4):24-32. [MEDLINE: ] - PubMed
References to studies awaiting assessment
NCT00440869 {published data only}
-
- Johansen KL. Effects of n-acetylcysteine on muscle fatigue in hemodialysis (NAC) [Effects of N-acetylcysteine on muscle fatigue in ESRD]. clinicaltrials.gov/ct2/show/NCT00440869 received 27 February 2007.
References to ongoing studies
ACTRN12617000420347 {published data only}
-
- Barr K. Evaluating the effectiveness of practicing yoga during haemodialysis for fatigue in patients with end stage kidney disease [Evaluating the effectiveness of intradialytic yoga for fatigue in patients with end stage kidney disease receiving haemodialysis: a mixed methods approach]. www.anzctr.org.au (first received 17 March 2017).
ACTRN12618000724279 {published data only}
-
- Chojak-Fijalka K. Evaluation of the effectiveness of home-based physical training in patients undergoing haemodialysis [The effects of home-based physical training in patients undergoing haemodialysis on physical functioning, body composition, quality of life, fatigue and selected properties of blood]. www.anzctr.org.au (first received 18 March 2018).
ACTRN12620000408987 {published data only}
-
- Tong A. Structured exercise prograM to reduce Fatigue In patients receiving dialysis: a preference-stratified adaptive Trial (M-FIT). www.anzctr.org.au 2020.
Burrai 2019a {published data only}
-
- Burrai F, Othman S, Brioni E, Micheluzzi V, Luppi M, Apuzzo L, et al. Effects of virtual reality in patients undergoing dialysis: study protocol. Holistic Nursing Practice 2019;33(6):327-37. [MEDLINE: ] - PubMed
Cardoso 2019 {published data only}
CONVINCE 2020 {published data only}
-
- Blankestijn PJ, Fischer KI, Barth C, Cromm K, Canaud B, Davenport A, et al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: the comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open 2020;10(2):e033228. [MEDLINE: ] - PMC - PubMed
-
- Fischer K, Blankestijn PJ, Cromm K, Bots M, Barth CM, Canaud B, et al. Assessment of generic and dialysis-related fatigue: identification of individual trajectories and investigation of ecological validity [abstract no: 3200]. Quality of Life Research 2020;29(Suppl 1):S180. [EMBASE: 634412125]
-
- Fischer KI, Blankestijn P, Cromm K, Bots M, Barth CM, Canaud B, et al. Assessing the patient's perspective in end-stage kidney disease - a domain-guided approach applied in the CONVINCE trial [abstract]. In: International Society for Quality of Life Research (ISOQOL); 26th Annual Conference; 2019 Oct 20-23, San Diego, CA. 2019. [https://www.ucl.ac.uk/convince-hemodiafiltration-dialysis-study/sites/co...]
-
- Fischer KI, Blankestijn PJ, Cromm K, Canaud BJ, Barth CM, Hegbrant JB, et al. A new approach to assess patient-reported outcomes of patients with ESKD in an international randomized clinical trial [abstract no: PUB117]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1104. [EMBASE: 633769665]
CTRI/2018/02/012021 {published data only}
-
- Raj PD, Bai RS. Exercise during dialysis for reducing tiredness and improving the sleep for patients undergoing hemodialysis [The effectiveness of intradialytic exercise on fatigue and quality of sleep among patients undergoing hemodialysis]. www.ctri.nic.in (first received 21 February 2018).
Hamad 2021 {published data only}
-
- Hamad AI, Mishra RK, Ibrahim RA, Mathew M, Mohamed MY, Ateya HM, et al. Effectiveness of intradialytic plantar electrical nerve stimulation during hemodialysis to improve the gait in adults with diabetes and renal failure: A randomized double-blinded controlled trial [abstract no: PO0773]. Journal of the American Society of Nephrology 2021;32:271. [EMBASE: 636328282]
-
- Mishra RK, Al-Ali F, Hamad A, Ibrahim R, Mathew M, Najafi B. Effect of plantar electrical nerve stimulation during routine hemodialysis process on the daily physical activity in adults with diabetes and endstage renal disease-a randomized doubleblinded controlled trial [abstract no: MO623). Nephrology Dialysis Transplantation 2021;36(Suppl 1):i367. [PMID: ] - PubMed
NCT01620580 {published data only}
-
- Danquah FV. Symptom management program for hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT01620580 (first received 24 October 2014).
NCT02361268 {published data only}
-
- Birdee GS. End-stage renal disease intra-dialysis lifestyle education study (END-IDLE). www.clinicalTrials.gov/show/NCT02361268 (first received 29 January 2015).
Quintiliano 2019 {published data only}
-
- Quintiliano A, Oehmen T, Kirsztajn GM, Pegado R. Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double-blind controlled randomized trial. Trials [Electronic Resource] 2019;20(1):805. [MEDLINE: ] - PMC - PubMed
Sharma 2022 {published data only}
SLEEP‐HD 2021 {published data only}
-
- McCurry S, Cukor D, Clark C, Brady N, Rue T, Unruh M, et al. Tailoring of cognitive behavior therapy for insomnia for patients with kidney failure undergoing hemodialysis: the sleep-HD study [abstract no: 359]. Sleep 2021;44(Suppl 2):A143. [EMBASE: 635915012]
TACcare 2018 {published data only}
-
- Devaraj SM, Yabes J, Roumelioti ME, Steel JL, Erickson SJ, Unruh ML, et al. Prevalence and demographic correlates of pain, depression, fatigue, and readiness to seek treatment for these symptoms in hemodialysis patients [abstract no: PO0834]. Journal of the American Society of Nephrology 2021;32:289. [EMBASE: 636330313]
-
- Roumelioti ME, Steel JL, Yabes J, Vowles KE, Vodovotz Y, Beach S, et al. Rationale and design of technology assisted stepped collaborative care intervention to improve patient-centered outcomes in hemodialysis patients (TACcare trial). Contemporary Clinical Trials 2018;73:81-91. [MEDLINE: ] - PMC - PubMed
van der Borg 2016 {published data only}
van der Veen 2021 {published data only}
-
- Veen Y, Post A, Kremer D, Westerhuis R, Franssen CF, Wallimann T, et al. A clinical approach of intradialytic creatine supplementation in dialysis-dependent CKD patients: a rationale and study design [abstract no: PO0858]. Journal of the American Society of Nephrology 2021;32:295. [EMBASE: 636331594]
Additional references
Artom 2014
-
- Artom M, Moss-Morris R, Caskey F, Chilcot J. Fatigue in advanced kidney disease. Kidney International 2014;86(3):497-505. [MEDLINE: ] - PubMed
Astroth 2013
-
- Astroth KS, Russell CL, Welch JL. Non-pharmaceutical fatigue interventions in adults receiving hemodialysis: a systematic review. Nephrology Nursing Journal 2013;40(5):407-27. [MEDLINE: ] - PubMed
Bergstrom 2000
-
- Bergström J, Lindholm B, Lacson E Jr, Owen W Jr, Lowrie EG, Glassock RJ, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Seminars in Dialysis 2000;13(3):163-75. [MEDLINE: ] - PubMed
Bossola 2020
-
- Bossola M, Di Stasio E, Monteburini T, Parodi E, Ippoliti F, Bonomini M, et al. Intensity, duration, and frequency of post-dialysis fatigue in patients on chronic haemodialysis. Journal of Renal Care 2020;46(2):115-23. [PMID: ] - PubMed
Bouya 2018
-
- Bouya S, Ahmadidarehsima S, Badakhsh M, Balouchi A, Koochakzai M. Effect of aromatherapy interventions on hemodialysis complications: a systematic review. Complementary Therapies in Clinical Practice 2018;32:130-8. [PMID: ] - PubMed
Chang 2001
-
- Chang WK, Hung KY, Huang JW, Wu KD, Tsai TJ. Chronic fatigue in long-term peritoneal dialysis patients. American Journal of Nephrology 2001;21(6):479-85. [MEDLINE: ] - PubMed
Chen 2008
-
- Chen HY, Chiang CK, Wang HH, Hung KY, Lee YJ, Peng YS, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. American Journal of Kidney Diseases 2008;52(2):314-23. [MEDLINE: ] - PubMed
Chertow 2021
-
- Chertow GM, Pergola PE, Farag YM, Agarwa lR, Arnold S, Bako G, et al. Vadadustat in patients with anemia and non dialysis-dependent CKD. New England Journal of Medicine 2021;384(17):1589-600. [PMID: ] - PubMed
Chiaranai 2016
-
- Chiaranai C. The lived experience of patients receiving hemodialysis treatment for end-stage renal disease: a qualitative study. Journal of Nursing Research 2016;24(2):101-8. [MEDLINE: ] - PubMed
Davey 2019
Debnath 2021
Eglence 2013
-
- Eglence R, Karatas, N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Alternative Therapies in Health & Medicine 2013;19(6):23-31. [MEDLINE: ] - PubMed
Evangelidis 2017
-
- Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. American Journal of Kidney Diseases 2017;70(4):464-75. [MEDLINE: ] - PubMed
Foley 2009
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Habibzadeh 2020
Higgins 2003
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, PageMJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Horigan 2013
Jhamb 2008
Johansen 2012
-
- Johansen KL, Finkelstein FO, Revicki DA, Evans C, Wan S, Gitlin M, et al. Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients. Nephrology Dialysis Transplantation 2012;27(6):2418-25. [MEDLINE: ] - PubMed
Ju 2018a
-
- Ju A, Unruh M, Davison S, Dapueto J, Dew MA, Fluck R, et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop Report. American Journal of Kidney Diseases 2018;72(1):104-12. [PMID: ] - PubMed
Ju 2018b
-
- Ju A, Unruh ML, Davison SN, Dapueto J, Dew MA, Fluck R, et al. Patient-reported outcome measures for fatigue in patients on hemodialysis: a systematic review. American Journal of Kidney Diseases 2018;71(3):327-43. [PMID: ] - PubMed
Ju 2021
Karadag 2019
-
- Karadag E, Samancioglu Baglama S. The effect of aromatherapy on fatigue and anxiety in patients undergoing hemodialysis treatment: a randomized controlled study. Holistic Nursing Practice 2019;33(4):222-9. [PMID: ] - PubMed
Lee 1991
-
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research 1991;36(3):291–8. [PMID: ] - PubMed
Liangos 2010
-
- Liangos O, Jaber BL. Chapter 24 - Acute complications associated with hemodialysis. In: Himmelfarb J, Sayegh MH, editors(s). Chronic Kidney Disease, Dialysis and Transplantation. 3rd edition. JB Saunders, 2010. [ISBN: 9781437709872]
Manera 2019
Maruyama 2021
Melo 2020
-
- Melo GA, Aguiar LL, Silva RA, Pereira FG, Silva FL, Caetano JA. Effects of acupuncture in patients with chronic kidney disease: a systematic review. Revista Brasileira de Enfermagem 2020;73(4):e20180784. [PMID: ] - PubMed
Monaro 2014
-
- Monaro S, Stewart G, Gullick J. A 'lost life': coming to terms with haemodialysis. Journal of Clinical Nursing 2014;23(21-22):3262-73. [MEDLINE: ] - PubMed
Ossareh 2003
-
- Ossareh S, Roozbeh J, Krishnan M, Liakopoulos V, Bargman JM, Oreopoulos DG. Fatigue in chronic peritoneal dialysis patients [Erratum in: Int Urol Nephrol. 2004;36(3):477]. International Urology & Nephrology 2003;35(4):535-41. [MEDLINE: ] - PubMed
Picariello 2017
-
- Picariello F, Hudson JL, Moss-Morris R, Macdougall IC, Chilcot J. Examining the efficacy of social-psychological interventions for the management of fatigue in end-stage kidney disease (ESKD): a systematic review with meta-analysis. Health Psychology Review 2017;11(2):197-216. [PMID: ] - PubMed
Picariello 2017a
Rao 2007
-
- Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney International 2007;72(5):549-56. [MEDLINE: ] - PubMed
Ross 2003
-
- Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clinical Therapeutics 2003;25(6):1786-805. [MEDLINE: ] - PubMed
Salehi 2020
Schreiber 2005
-
- Schreiber B. Levocarnitine and dialysis: a review. Nutrition in Clinical Practice 2005;20(2):218-43. [MEDLINE: ] - PubMed
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, ChandlerJ, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Song 2018
-
- Song YY, Hu RJ, Diao YS, Chen L, Jian XI. Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. Journal of Pain & Symptom Management 2018;55(4):1184-95. [PMID: ] - PubMed
SONG‐HD
-
- Standardised Outcomes in Nephrology - Haemodialysis. https://songinitiative.org/projects/song-hd/ (date accessed 17 July 2023).
SONG‐PD
-
- Standardised Outcomes in Nephrology - Peritoneal Dialysis. https://songinitiative.org/projects/song-pd/ (date accessed 17 July 2023).
Tian 2020
-
- Tian C, Zhang B, Liang W, Yang Q, Xiong Q, Jin Q, et al. Fatigue in peritoneal dialysis patients and an exploration of contributing factors: a cross-sectional study. Journal of Pain & Symptom Management 2020;59(5):1074-81. [PMID: ] - PubMed
Tong 2017
-
- Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, et al. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. American Journal of Kidney Diseases 2017;69(1):97-107. [MEDLINE: ] - PMC - PubMed
Unruh 2020
Urquhart‐Secord 2016
-
- Urquhart-Secord R, Craig JC, Hemmelgarn B, Tam-Tham H, Manns B, Howell M, et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. American Journal of Kidney Diseases 2016;68(3):444-54. [MEDLINE: ] - PubMed
van Sandwijk 2019
-
- Sandwijk MS, Al Arashi D, de Hare FM, Torren JM, Kersten MJ, Bijlsma JA, et al. Fatigue, anxiety, depression and quality of life in kidney transplant recipients, haemodialysis patients, patients with a haematological malignancy and healthy controls. Nephrology Dialysis Transplantation 2019;34(5):833–8. [PMID: ] - PubMed
Yngman‐Uhlin 2010
-
- Yngman-Uhlin P, Friedrichsen M, Gustavsson M, Fernstrom A, Edell-Gustaffson U. Circling around in tiredness: perspectives of patients on peritoneal dialysis. Nephrology Nursing Journal 2010;37(4):407-13. [MEDLINE: ] - PubMed
Yurtkuran 2007
-
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complementary Therapies in Medicine 2007;15(3):164-71. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

