A nationwide pharmacovigilance investigation on trends and seriousness of adverse events induced by anti-obesity medication
- PMID: 37651636
- PMCID: PMC10471157
- DOI: 10.7189/jogh.13.04095
A nationwide pharmacovigilance investigation on trends and seriousness of adverse events induced by anti-obesity medication
Abstract
Introduction: Despite rising concerns regarding the safety of anti-obesity medications, there is a lack of comprehensive pharmacovigilance investigations utilising real-world data. We aimed to characterise the prevalence and seriousness of adverse drug events (ADEs) related to anti-obesity medications and to identify predictors associated with increased risk of serious adverse events (SAE), thereby conveying evidence on drug safety.
Methods: We conducted a cross-sectional analysis on ADE cases spontaneously reported to the Korea Adverse Event Reporting System Database (KIDS-KD). ADE reports pertaining to anti-obesity medications prescribed for overweight, obesity (International Classification of Disease, 10th revision (ICD-10) code E66) and abnormal weight gain (ICD-10 code E63.5) were included in the analysis. We performed a disproportionality to detect the association of the system organ class-based ADEs with their seriousness an individual's sex by estimating reporting odds ratios (RORs) and their 95% confidence intervals (CIs). We performed logistic regression to investigate factors that are substantially associated with increased SAE risks by estimating odds ratio (OR) and their 95% CIs.
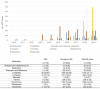
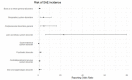
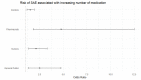
Results: The most common causative anti-obesity medication was phentermine, followed by liraglutide. ADEs associated with psychiatric disorders (ROR = 1.734; 95% CI = 1.111-2.707), liver and biliary system disorders (ROR = 22.948; 95% CI = 6.613-70.635), cardiovascular disorders (ROR = 5.707; 95% CI = 1.965-16.574), and respiratory disorders (ROR = 4.567; 95% CI = 1.774-11.762) were more likely to be serious events. Additionally, men are more likely to experience ADEs related gastrointestinal disorders (ROR = 1.411) and less likely to have heart and rhythm disorders (ROR = 0.507). The risk of SAE incidences was positively correlated with being male (OR = 2.196; 95% CI = 1.296-3.721), dual or triple combination of anti-obesity medications (OR = 3.258; 95% CI = 1.633-6.501 and OR = 8.226; 95% CI = 3.046-22.218, respectively), and concomitant administration of fluoxetine (OR = 5.236; 95% CI = 2.218-12.365).
Conclusions: Seriousness of anti-obesity medication-related ADEs differs among system-organ class, while sex-related differences in ADE profiles are also present. The predictors substantially increasing risk of SAE incidences include being male, having a higher number of concomitant medications (including multiple combination of anti-obesity medications), and concurrent use of fluoxetine. Nonetheless, further pharmacovigilance investigation and monitoring are needed to enhance awareness on ADEs induced by anti-obesity medications.
Copyright © 2023 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Figures

Similar articles
-
A Comprehensive 10-Year Nationwide Pharmacovigilance Surveillance on Antibacterial Agents in Korea: Data Mining for Signal Detection of Trends and Seriousness of Adverse Events.Microorganisms. 2025 Jan 10;13(1):136. doi: 10.3390/microorganisms13010136. Microorganisms. 2025. PMID: 39858904 Free PMC article.
-
A Real-World Data Derived Pharmacovigilance Assessment on Drug-Induced Nephropathy: Implication on Gaps in Patient Care.Healthcare (Basel). 2023 Dec 31;12(1):95. doi: 10.3390/healthcare12010095. Healthcare (Basel). 2023. PMID: 38201001 Free PMC article.
-
Real-World Data-Derived Pharmacovigilance on Drug-Induced Cognitive Impairment Utilizing a Nationwide Spontaneous Adverse Reporting System.Medicina (Kaunas). 2024 Jun 23;60(7):1028. doi: 10.3390/medicina60071028. Medicina (Kaunas). 2024. PMID: 39064457 Free PMC article.
-
Risk factors of immune checkpoint inhibitor-associated acute kidney injury: evidence from clinical studies and FDA pharmacovigilance database.BMC Nephrol. 2023 Apr 22;24(1):107. doi: 10.1186/s12882-023-03171-9. BMC Nephrol. 2023. PMID: 37087434 Free PMC article.
-
Cardiovascular safety of rapidly accelerated fibrosarcoma B-type and/or mitogen-activated extracellular signal-regulated kinase inhibitors: A mixed approach combining a meta-analysis and a pharmacovigilance disproportionality analysis.Arch Cardiovasc Dis. 2020 Jun-Jul;113(6-7):420-432. doi: 10.1016/j.acvd.2020.03.014. Epub 2020 May 1. Arch Cardiovasc Dis. 2020. PMID: 32418884
Cited by
-
A Comprehensive 10-Year Nationwide Pharmacovigilance Surveillance on Antibacterial Agents in Korea: Data Mining for Signal Detection of Trends and Seriousness of Adverse Events.Microorganisms. 2025 Jan 10;13(1):136. doi: 10.3390/microorganisms13010136. Microorganisms. 2025. PMID: 39858904 Free PMC article.
-
Investigation into Safety Profiles of Antiepileptic Drugs and Identification of Predictors for Serious Adverse Events: Insights from National Pharmacovigilance Data.Pharmaceuticals (Basel). 2025 Jul 7;18(7):1013. doi: 10.3390/ph18071013. Pharmaceuticals (Basel). 2025. PMID: 40732301 Free PMC article.
-
The anti-obesity effects of polyphenols: a comprehensive review of molecular mechanisms and signal pathways in regulating adipocytes.Front Nutr. 2024 Oct 30;11:1393575. doi: 10.3389/fnut.2024.1393575. eCollection 2024. Front Nutr. 2024. PMID: 39539361 Free PMC article. Review.
-
A Real-World Data Driven Pharmacovigilance Investigation on Drug-Induced Arrhythmia Using KAERS DB, a Korean Nationwide Adverse Drug Reporting System.Pharmaceuticals (Basel). 2023 Nov 15;16(11):1612. doi: 10.3390/ph16111612. Pharmaceuticals (Basel). 2023. PMID: 38004477 Free PMC article.
-
A Real-World Data Derived Pharmacovigilance Assessment on Drug-Induced Nephropathy: Implication on Gaps in Patient Care.Healthcare (Basel). 2023 Dec 31;12(1):95. doi: 10.3390/healthcare12010095. Healthcare (Basel). 2023. PMID: 38201001 Free PMC article.
References
-
- Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi’i A.A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med. 2021;136:104754. 10.1016/j.compbiomed.2021.104754 - DOI - PubMed
-
- World Health Organization. Obesity and overweight. 2021. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed: 9 June 2021.
-
- Centers for Disease Control and Prevention. Adult obesity facts. 2022. Available: https://www.cdc.gov/obesity/data/adult.html. Accessed: 2 May 2023.
-
- Centers for Disease Control and Prevention. Obesity and cancer. 2023. Available: https://www.cdc.gov/cancer/obesity/index.htm. Accessed: 2 May 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
