Real-World Use of Positron Emission Tomography-Computed Tomography and Reported Deauville Scores in Advanced-Stage Classic Hodgkin Lymphoma: A Community Oncology Practice Perspective
- PMID: 37651672
- PMCID: PMC10615434
- DOI: 10.1200/OP.23.00021
Real-World Use of Positron Emission Tomography-Computed Tomography and Reported Deauville Scores in Advanced-Stage Classic Hodgkin Lymphoma: A Community Oncology Practice Perspective
Abstract
Purpose: To evaluate the use of interim positron emission tomography-computed tomography (PET-CT) scans and Deauville 5-point scale (5PS) score reporting for stage III/IV classic Hodgkin lymphoma (cHL) treated frontline (1L) in community oncology settings.
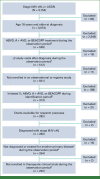
Methods: This retrospective, observational study included adults with stage III/IV cHL initiating 1L doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine, or an escalated dosing regimen of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone within the US Oncology Network between January 2017 and October 2019. Data were collected from electronic health records and chart reviews and summarized descriptively.
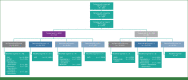
Results: A total of 262 patients were included; 48.9% were age 39 years or younger. Most were male (57%), White (59%), had an International Prognostic Score <4 (76%), and received 1L ABVD (74%). Forty-nine percent of patients had stage III and 51% had stage IV cHL. Of 258 patients with ≥1 PET-CT scan, 71% (n = 184) had an interim scan and 64% received ≥1 scan at an off-site location. Of patients treated 1L with ABVD who received a baseline and interim scan, Deauville 5PS scores were not documented for 45% of patients; in 90% of these cases, a standardized uptake value (SUV) was reported.
Conclusion: In community oncology settings, under-reporting of Deauville 5PS scores for interim PET-CT scans was observed. In the absence of Deauville 5PS scores, SUV results were generally provided. These results highlight educational opportunities that exist for PET-adapted ABVD, including consistency in reporting/utilization of Deauville 5PS scores to de-escalate or escalate treatment.
Conflict of interest statement
Disclosures provided by the authors are available with this article at DOI
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Ansell SM: Hodgkin lymphoma: A 2020 update on diagnosis, risk-stratification, and management. Am J Hematol 95:978-989, 2020 - PubMed
-
- National Cancer Institute : SEER cancer statistics review (CSR) 1975-2015. https://seer.cancer.gov/archive/csr/1975_2015/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

