Efficacy and Safety of Approximately 3 Years of Continuous Ozanimod in Moderately to Severely Active Ulcerative Colitis: Interim Analysis of the True North Open-label Extension
- PMID: 37651686
- PMCID: PMC10896634
- DOI: 10.1093/ecco-jcc/jjad146
Efficacy and Safety of Approximately 3 Years of Continuous Ozanimod in Moderately to Severely Active Ulcerative Colitis: Interim Analysis of the True North Open-label Extension
Abstract
Backgrounds and aims: This interim analysis from the True North open-label extension [OLE] study examines efficacy and safety of approximately 3 years of continuous ozanimod treatment in patients with moderately to severely active ulcerative colitis.
Methods: Clinical responders after 52 weeks of ozanimod during the phase 3 True North study, who continued treatment in the OLE, were evaluated. Efficacy, including endoscopic and histological endpoints, was assessed during the OLE for approximately 2 additional years through OLE Week 94, using observed case [OC] and nonresponder imputation [NRI] analyses. Adverse events were monitored from True North baseline through OLE data cutoff and expressed as exposure-adjusted incidence rates.
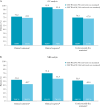
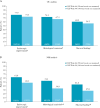
Results: This analysis included 131 patients; 54% had achieved corticosteroid-free remission at True North Week 52. In OC analyses, clinical response, clinical remission, and corticosteroid-free remission were achieved by 91.4%, 69.1%, and 67.9% of patients, respectively, at OLE Week 94 [146 weeks of total treatment]. Similarly, endoscopic improvement, histological remission, and mucosal healing were achieved by 73.3%, 67.3%, and 56.3% of patients, respectively, at OLE Week 94. Efficacy rates were lower using NRI analyses, but maintenance of efficacy was demonstrated through OLE Week 94. No new safety signals emerged from this analysis. Serious infections, malignancy, cardiovascular events, and hepatic events occurred infrequently.
Conclusions: Among patients who achieved clinical response after 1 year of ozanimod treatment during True North, a high percentage sustained clinical and mucosal efficacy over 2 additional years in the OLE. No new safety signals were observed with long-term ozanimod use.
Keywords: S1P receptor modulator; Ulcerative colitis; ozanimod.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
Figures


References
-
- Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med 2020;383:2652–64. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. .; International Organization for the Study of IBD. Stride-II: An update on the selecting therapeutic targets in inflammatory bowel disease [STRIDE initiative of the International Organization for the Study of IBD [IOIBD]: determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. - PubMed
-
- Zeposia [summary of product characteristics]. Utrecht, Netherlands: Celgene Distribution B.V.; 2023.
-
- Zeposia [package insert]. Princeton, N.J.: Bristol Myers Squibb; 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

