Scope and Mechanistic Probe into Asymmetric Synthesis of α-Trisubstituted-α-Tertiary Amines by Rhodium Catalysis
- PMID: 37651695
- PMCID: PMC10581542
- DOI: 10.1021/jacs.3c04211
Scope and Mechanistic Probe into Asymmetric Synthesis of α-Trisubstituted-α-Tertiary Amines by Rhodium Catalysis
Abstract
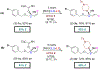
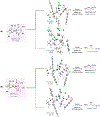
Asymmetric reactions that convert racemic mixtures into enantioenriched amines are of significant importance due to the prevalence of amines in pharmaceuticals, with about 60% of drug candidates containing tertiary amines. Although transition-metal catalyzed allylic substitution processes have been developed to provide access to enantioenriched α-disubstituted allylic amines, enantioselective synthesis of sterically demanding α-tertiary amines with a tetrasubstituted carbon stereocenter remains a major challenge. Herein, we report a chiral diene-ligated rhodium-catalyzed asymmetric substitution of racemic tertiary allylic trichloroacetimidates with aliphatic secondary amines to afford α-trisubstituted-α-tertiary amines. Mechanistic investigation is conducted using synergistic experimental and computational studies. Density functional theory calculations show that the chiral diene-ligated rhodium promotes the ionization of tertiary allylic substrates to form both anti and syn π-allyl intermediates. The anti π-allyl pathway proceeds through a higher energy than the syn π-allyl pathway. The rate of conversion of the less reactive π-allyl intermediate to the more reactive isomer via π-σ-π interconversion was faster than the rate of nucleophilic attack onto the more reactive intermediate. These data imply that the Curtin-Hammett conditions are met in the amination reaction, leading to dynamic kinetic asymmetric transformation. Computational studies also show that hydrogen bonding interactions between β-oxygen of allylic substrate and amine-NH greatly assist the delivery of amine nucleophile onto more hindered internal carbon of the π-allyl intermediate. The synthetic utility of the current methodology is showcased by efficient preparation of α-trisubstituted-α-tertiary amines featuring pharmaceutically relevant secondary amine cores with good yields and excellent selectivities (branched-linear >99:1, up to 99% enantiomeric excess).
Conflict of interest statement
The authors declare no competing financial interest.
Figures
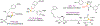
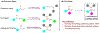
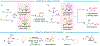

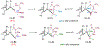

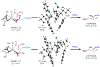


References
-
- Hager A; Vrielink N; Hager D; Lefranc J; Trauner D Synthetic approaches towards alkaloids bearing alpha-tertiary amines. Nat. Prod. Rep 2016, 33, 491–522. - PubMed
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
- Trowbridge A; Walton SM; Gaunt MJ New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692. - PubMed
-
- Roughley SD; Jordan AM The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem 2011, 54, 3451–3479. - PubMed
-
- Morgenthaler M Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem 2007, 2, 1100–1115. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

