Mechanotransduction: Forcing a change in metabolism
- PMID: 37651955
- PMCID: PMC10523412
- DOI: 10.1016/j.ceb.2023.102219
Mechanotransduction: Forcing a change in metabolism
Abstract
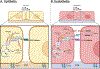
Epithelial and endothelial cells experience numerous mechanical cues throughout their lifetimes. Cells resist these forces by fortifying their cytoskeletal networks and adhesions. This reinforcement is energetically costly. Here we describe how these energetic demands are met. We focus on the response of epithelial and endothelial cells to mechanical cues, describe the energetic needs of epithelia and endothelia, and identify the mechanisms these cells employ to increase glycolysis, oxidative phosphorylation, and fatty acid metabolism. We discuss the similarities and differences in the responses of the two cell types.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Figures
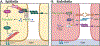

References
-
- Zuidema A, Wang W, and Sonnenberg A, Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays, 2020. 42(11): p. 2000119. - PubMed
-
- Charras G. and Yap AS, Tensile Forces and Mechanotransduction at Cell–Cell Junctions. Current Biology, 2018. 28(8): p. R445–R457. - PubMed
-
- Demali KA, Sun X, and Bui GA, Force Transmission at Cell–Cell and Cell–Matrix Adhesions. Biochemistry, 2014. 53(49): p. 7706–7717. - PubMed
-
- Manibog K, et al., Resolving the molecular mechanism of cadherin catch bond formation. Nature Communications, 2014. 5(1). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

