Inhalable Nanofitin demonstrates high neutralization of SARS-CoV-2 virus via direct application in respiratory tract
- PMID: 37652011
- PMCID: PMC10556219
- DOI: 10.1016/j.ymthe.2023.08.010
Inhalable Nanofitin demonstrates high neutralization of SARS-CoV-2 virus via direct application in respiratory tract
Abstract
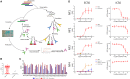
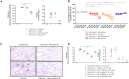
Nanofitins are small and hyperthermostable alternative protein scaffolds that display physicochemical properties making them suitable for the development of topical therapeutics, notably for the treatment of pulmonary infectious diseases. Local administration of biologics to the lungs involves a particularly stressful step of nebulization that is poorly tolerated by most antibodies, which limits their application by this delivery route. During the COVID-19 pandemic, we generated anti-SARS-CoV-2 monomeric Nanofitins of high specificity for the spike protein. Hit Nanofitin candidates were identified based on their binding properties with punctual spike mutants and assembled into a linear multimeric construction constituting of four different Nanofitins, allowing the generation of a highly potent anti-SARS-CoV-2 molecule. The therapeutic efficacy of the multimeric assembly was demonstrated both in in vitro and in vivo models. Interestingly, the neutralization mechanism of the multimeric construction seems to involve a particular conformation switch of the spike trimer. In addition, we reported the stability and the conserved activity of the tetrameric construction after nebulization. This advantageous developability feature for pulmonary administration associated with the ease of assembly, as well as the fast generation process position the Nanofitin technology as a potential therapeutic solution for emerging infectious diseases.
Keywords: Nanofitin; antibody mimetic; infectious disease; inhalable; nebulization; protein scaffold; pulmonary delivery.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The Nanofitin technology described in this study, commercialized by Affilogic, uses the patent application owned by Institut Pasteur and Center National de la Recherche Scientifique (CNRS): “OB-fold used as scaffold for engineering new specific binders”; PCT/IB2007/004388. Affilogic SAS, Nantes, France, provided support for the study and participated in study design, conducted the study, and provided data collection, management and interpretation. S.V., E.E, J.P., L.N., M.C., S.H., are employees of Affilogic SAS. O.K. is the CEO and the owner of Affilogic SAS.
Figures





References
-
- Kumari M., Lu R.-M., Li M.-C., Huang J.-L., Hsu F.-F., Ko S.-H., Ke F.-Y., Su S.-C., Liang K.-H., Yuan J.P.-Y., et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J. Biomed. Sci. 2022;29:68. doi: 10.1186/s12929-022-00852-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

