Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer's disease via MFG-E8
- PMID: 37652017
- PMCID: PMC10518633
- DOI: 10.1016/j.xcrm.2023.101175
Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer's disease via MFG-E8
Abstract
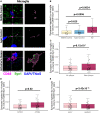
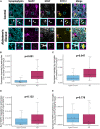
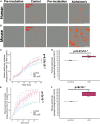
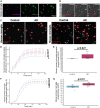
Synapse loss correlates with cognitive decline in Alzheimer's disease (AD). Data from mouse models suggests microglia are important for synapse degeneration, but direct human evidence for any glial involvement in synapse removal in human AD remains to be established. Here we observe astrocytes and microglia from human brains contain greater amounts of synaptic protein in AD compared with non-disease controls, and that proximity to amyloid-β plaques and the APOE4 risk gene exacerbate this effect. In culture, mouse and human astrocytes and primary mouse and human microglia phagocytose AD patient-derived synapses more than synapses from controls. Inhibiting interactions of MFG-E8 rescues the elevated engulfment of AD synapses by astrocytes and microglia without affecting control synapse uptake. Thus, AD promotes increased synapse ingestion by human glial cells at least in part via an MFG-E8 opsonophagocytic mechanism with potential for targeted therapeutic manipulation.
Keywords: Alzheimer’s disease; MFGE8; astrocytes; microglia; synapse loss; synapses.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.L.S.-J. is on the Scientific Advisory Board of Cognition Therapeutics and Scottish Brain Sciences and receives collaborative grant funding from two industry partners. None of these had any influence over the current paper.
Figures



References
-
- Pickett E.K., Herrmann A.G., McQueen J., Abt K., Dando O., Tulloch J., Jain P., Dunnett S., Sohrabi S., Fjeldstad M.P., et al. Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep. 2019;29:3592–3604.e5. doi: 10.1016/j.celrep.2019.11.044. - DOI - PMC - PubMed
-
- Colom-Cadena M., Davies C., Sirisi S., Lee J.-E., Simzer E.M., Tzioras M., Querol-Vilaseca M., Sánchez-Aced É., Chang Y.Y., Holt K., et al. Synaptic oligomeric tau in Alzheimer’s disease — A potential culprit in the spread of tau pathology through the brain. Neuron. 2023;111:2170–2183.e6. doi: 10.1016/j.neuron.2023.04.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

