Mural cell-derived chemokines provide a protective niche to safeguard vascular macrophages and limit chronic inflammation
- PMID: 37652021
- PMCID: PMC10588993
- DOI: 10.1016/j.immuni.2023.08.002
Mural cell-derived chemokines provide a protective niche to safeguard vascular macrophages and limit chronic inflammation
Abstract
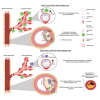
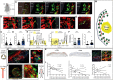
Maladaptive, non-resolving inflammation contributes to chronic inflammatory diseases such as atherosclerosis. Because macrophages remove necrotic cells, defective macrophage programs can promote chronic inflammation with persistent tissue injury. Here, we investigated the mechanisms sustaining vascular macrophages. Intravital imaging revealed a spatiotemporal macrophage niche across vascular beds alongside mural cells (MCs)-pericytes and smooth muscle cells. Single-cell transcriptomics, co-culture, and genetic deletion experiments revealed MC-derived expression of the chemokines CCL2 and MIF, which actively preserved macrophage survival and their homeostatic functions. In atherosclerosis, this positioned macrophages in viable plaque areas, away from the necrotic core, and maintained a homeostatic macrophage phenotype. Disruption of this MC-macrophage unit via MC-specific deletion of these chemokines triggered detrimental macrophage relocalizing, exacerbated plaque necrosis, inflammation, and atheroprogression. In line, CCL2 inhibition at advanced stages of atherosclerosis showed detrimental effects. This work presents a MC-driven safeguard toward maintaining the homeostatic vascular macrophage niche.
Keywords: CCL2; MIF; atherosclerosis; chemokines; chronic inflammation; macrophages; mural cells; pericytes; smooth muscle cells; vascular macrophages.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Moderna, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. P.L. is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Eulicid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, Dewpoint, Plaque Tec, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech, Inc. P.L.'s laboratory has received research funding in the last 2 years from Novartis, Novo Nordisk, and Genentech. P.L. is on the Board of Directors of XBiotech, Inc. P.L. has a financial interest in Xbiotech (a company developing therapeutic human antibodies), in TenSixteen Bio (a company targeting somatic mosaicism and clonal hematopoiesis of indeterminate potential [CHIP] to discover and develop novel therapeutics to treat age-related diseases), and in Soley Therapeutics (a biotechnology company that is combining artificial intelligence with molecular and cellular response detection for discovering and developing new drugs, currently focusing on cancer therapeutics). P.L.'s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

