Nitric oxide and thioredoxin modulate the activity of caspase 9 in HepG2 cells
- PMID: 37652366
- PMCID: PMC10592080
- DOI: 10.1016/j.bbagen.2023.130452
Nitric oxide and thioredoxin modulate the activity of caspase 9 in HepG2 cells
Abstract
The interdependent and finely tuned balance between the well-established redox-based modification, S-nitrosylation, and its counteractive mechanism of S-nitrosothiol degradation, i.e., S-denitrosylation of biological protein or non-protein thiols defines the cellular fate in the context of redox homeostasis. S-nitrosylation of cysteine residues by S-nitrosoglutathione, S-nitroso-L-cysteine-like physiological and S-nitroso-L-cysteine ethyl ester-like synthetic NO donors inactivate Caspase-3, 8, and 9, thereby hindering their apoptotic activity. However, spontaneous restoration of their activity upon S-denitrosylation of S-nitrosocaspases into their reduced, free thiol active states, aided by the members of the ubiquitous cellular redoxin (thioredoxin/ thioredoxin reductase/ NADPH) and low molecular weight dithiol (lipoic acid/ lipoamide dehydrogenase/ dihydrolipoic acid/ NADPH) systems imply a direct relevance to their proteolytic activities and further downstream signaling cascades. Additionally, our previous and current findings offer crucial insight into the concept of redundancy between thioredoxin and lipoic acid systems, and the redox-modulated control of the apoptotic and proteolytic activity of caspases, triggering their cyto- and neurotoxic effects in response to nitro-oxidative stress. Thus, this might lay the foundation for the exogenous introduction of precise and efficient NO or related donor drug delivery systems that can directly participate in catering to the S-(de)-nitrosylation-mediated functional outcomes of the cysteinyl proteases in pathophysiological settings.
Keywords: Caspase 9; Lipoic acid; Nitric oxide; S-nitrosylation; Thioredoxin.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures
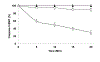

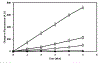

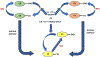
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

