Serious Immune-related Adverse Events Are Associated With Greater Efficacy of Nivolumab Therapy Against Non-small Cell Lung Cancer
- PMID: 37652490
- PMCID: PMC10500521
- DOI: 10.21873/invivo.13324
Serious Immune-related Adverse Events Are Associated With Greater Efficacy of Nivolumab Therapy Against Non-small Cell Lung Cancer
Abstract
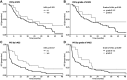
Background/aim: The aim of this study was to investigate possible association between adverse events of nivolumab therapy and the effectiveness of treatment in patients with non-small cell lung cancer (NSCLC). Focusing on serious adverse events (i.e., those of grade ≥3), we evaluated overall survival (OS), progression-free survival (PFS), as well as objective response rate (ORR) to treatment.
Patients and methods: We retrospectively analyzed a set of patients from the TULUNG database of NSCLC treated with nivolumab in eight oncology centers. We evaluated OS data based upon this set. To reduce possible bias, we further evaluated a subgroup of patients treated at the University Hospital in Pilsen, where the occurrence of adverse events, PFS, and ORR were independently examined by two experienced physicians. Survival statistics were evaluated using the Kaplan-Meier method and Cox analysis.
Results: We observed significantly greater OS, PFS, and ORR in the group of patients experiencing adverse events upon nivolumab treatment versus in those patients without such events. Although the univariable model analyzing the data set of all patients demonstrated higher OS in patients with serious adverse events, only a nonsignificant trend was observed in the Cox multivariable model. In a subgroup of patients with PFS and ORR evaluation, we did observe significant, favorable effects for patients having had serious adverse effects.
Conclusion: Patients experiencing severe adverse events show a tendency toward better OS, PFS, and ORR compared to patients without or having only mild adverse events with nivolumab treatment.
Keywords: Lung cancer; adverse events; nivolumab; overall survival.
Copyright © 2023, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare, regarding the publication of the article, that they provided consulting services to the company BMS and had scientific/educational events co-financed by this company. Marek Stastny is an employee of BMS in the position of Medical Advisor.
Figures

References
-
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D’Amico TA, Dilling TJ, Dowell J, Gettinger S, Gubens MA, Hegde A, Hennon M, Lackner RP, Lanuti M, Leal TA, Lin J, Loo BW Jr, Lovly CM, Martins RG, Massarelli E, Morgensztern D, Ng T, Otterson GA, Patel SP, Riely GJ, Schild SE, Shapiro TA, Singh AP, Stevenson J, Tam A, Yanagawa J, Yang SC, Gregory KM, Hughes M. NCCN guidelines insights: Non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–266. doi: 10.6004/jnccn.2021.0013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
