Atezolizumab Monotherapy for Non-small Cell Lung Cancer Patients: An Observational Study in Ibaraki Group (ATTENTION-IBARAKI)
- PMID: 37652502
- PMCID: PMC10500511
- DOI: 10.21873/invivo.13320
Atezolizumab Monotherapy for Non-small Cell Lung Cancer Patients: An Observational Study in Ibaraki Group (ATTENTION-IBARAKI)
Abstract
Background/aim: Atezolizumab is a monoclonal antibody that targets programmed death-ligand 1 (PD-L1) expressed on cancer cells derived from various organs and antigen-presenting cells and is currently commonly used in combination with chemotherapy. We conducted a study to clarify the current status of response to atezolizumab monotherapy in clinical practice and clarify the factors that contribute to long-term response and survival.
Patients and methods: We conducted a retrospective review of patients with advanced non-small cell lung cancer (NSCLC) treated with atezolizumab monotherapy from April 2018 to March 2023 at 11 Hospitals.
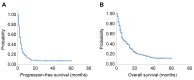
Results: The 147 patients evaluated had a progression-free survival (PFS) of 3.0 months and an overall survival of 7.0 months. Immune-related adverse events of any grade were observed in 13 patients (8.8%), grade 3 or higher in nine patients (6.1%), and grade 5 with pulmonary toxicity in one patient (0.7%). Favorable factors related to PFS were 'types of NSCLC other than adenocarcinoma'. Favorable factors for overall survival were 'performance status 0-1' and 'treatment lines up to 3'. There were 16 patients (10.9%) with PFS >1 year. No characteristic clinical findings were found in these 16 patients compared to the remaining 131 patients.
Conclusion: Efficacy and immune-related adverse events of NSCLC patients associated with atezolizumab monotherapy were comparable to those of previous clinical trial results. Knowledge of characteristics of patients who are most likely to benefit from atezolizumab monotherapy is a crucial step towards implementing appropriate prescribing.
Keywords: Atezolizumab; chemotherapy; immune-related adverse events; overall survival; progression-free survival.
Copyright © 2023, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to declare in relation to this study.
Figures
Similar articles
-
Atezolizumab Monotherapy or Plus Chemotherapy in First-Line Treatment for Advanced Non-Small Cell Lung Cancer Patients: A Meta-Analysis.Front Immunol. 2021 Jun 2;12:666909. doi: 10.3389/fimmu.2021.666909. eCollection 2021. Front Immunol. 2021. PMID: 34149702 Free PMC article.
-
Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study.Lancet Oncol. 2022 Jun;23(6):781-792. doi: 10.1016/S1470-2045(22)00226-1. Epub 2022 May 13. Lancet Oncol. 2022. PMID: 35576957 Clinical Trial.
-
Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC.N Engl J Med. 2020 Oct 1;383(14):1328-1339. doi: 10.1056/NEJMoa1917346. N Engl J Med. 2020. PMID: 32997907 Clinical Trial.
-
Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer.J Immunother Cancer. 2021 Mar;9(3):e001865. doi: 10.1136/jitc-2020-001865. J Immunother Cancer. 2021. PMID: 33737339 Free PMC article. Clinical Trial.
-
The safety of atezolizumab plus chemotherapy for the treatment of metastatic lung cancer.Expert Opin Drug Saf. 2020 Jul;19(7):775-783. doi: 10.1080/14740338.2020.1767584. Epub 2020 May 26. Expert Opin Drug Saf. 2020. PMID: 32400223 Review.
References
-
- Gandara DR, Von Pawel J, Mazieres J, Sullivan R, Helland Å, Han J, Ponce Aix S, Rittmeyer A, Barlesi F, Kubo T, Park K, Goldschmidt J, Gandhi M, Yun C, Yu W, Matheny C, He P, Sandler A, Ballinger M, Fehrenbacher L. Atezolizumab treatment beyond progression in advanced NSCLC: Results from the randomized, phase III OAK study. J Thorac Oncol. 2018;13(12):1906–1918. doi: 10.1016/j.jtho.2018.08.2027. - DOI - PubMed
-
- Herbst RS, Giaccone G, De Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, Mccleland M, Mocci S, Jassem J, Spigel DR. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
