Membrane homeostasis beyond fluidity: control of membrane compressibility
- PMID: 37652754
- PMCID: PMC10580326
- DOI: 10.1016/j.tibs.2023.08.004
Membrane homeostasis beyond fluidity: control of membrane compressibility
Abstract
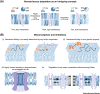
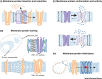
Biomembranes are complex materials composed of lipids and proteins that compartmentalize biochemistry. They are actively remodeled in response to physical and metabolic cues, as well as during cell differentiation and stress. The concept of homeoviscous adaptation has become a textbook example of membrane responsiveness. Here, we discuss limitations and common misconceptions revolving around it. By highlighting key moments in the life cycle of a transmembrane protein, we illustrate that membrane thickness and a finely regulated membrane compressibility are crucial to facilitate proper membrane protein insertion, function, sorting, and inheritance. We propose that the unfolded protein response (UPR) provides a mechanism for endoplasmic reticulum (ER) membrane homeostasis by sensing aberrant transverse membrane stiffening and triggering adaptive responses that re-establish membrane compressibility.
Keywords: homeoviscous adaptation; membrane compressibility; membrane fluidity; membrane thickness; unfolded protein response.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

