Low intensity pulsed ultrasound ameliorates Adriamycin-induced chronic renal injury by inhibiting ferroptosis
- PMID: 37652897
- PMCID: PMC10472869
- DOI: 10.1080/13510002.2023.2251237
Low intensity pulsed ultrasound ameliorates Adriamycin-induced chronic renal injury by inhibiting ferroptosis
Abstract
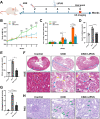
Objective: It is very important to develop a new therapeutic strategy to cope with the increasing morbidity and mortality of chronic kidney disease (CKD). As a kind of physical therapy, low intensity pulsed ultrasound (LIPUS) has remarkable anti-inflammatory and repair-promoting effects and is expected to become a new therapeutic method for CKD. This study aims to clarify the treatment effect of LIPUS on CKD-related renal inflammation and fibrosis, and to further explore the potential signal network of LIPUS treatment for ameliorating chronic renal injury.
Methods: A rat model simulating the progress of CKD was established by twice tail-vein injection of Adriamycin (ADR). Under anesthesia, bilateral kidneys of CKD rats were continuously stimulated by LIPUS for four weeks. The parameters of LIPUS were 1.0 MHz, 60 mW/cm2, 50% duty cycle and 20 min/d.
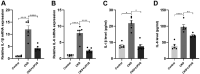
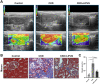
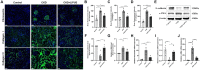
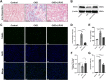
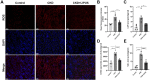
Results: LIPUS treatment effectively inhibited ADR-induced renal inflammation and fibrosis, and improved CKD-related to oxidative stress and ferroptosis. In addition, the therapeutic effect of LIPUS is closely related to the regulation of TGF-β1/Smad and Nrf2/keap1/HO-1 signalling pathways.
Discussion: This study provides a new direction for further mechanism research and lays an important foundation for clinical trials.
Keywords: Chronic kidney disease; Ferroptosis; Fibrosis; Inflammation; Low intensity pulsed ultrasound; Nrf2/keap1/HO-1 pathway; Oxidative stress; TGF-β1/Smad pathway.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

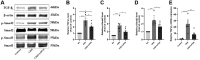


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
