Antibody-mediated neutralization of galectin-3 as a strategy for the treatment of systemic sclerosis
- PMID: 37652913
- PMCID: PMC10471779
- DOI: 10.1038/s41467-023-41117-9
Antibody-mediated neutralization of galectin-3 as a strategy for the treatment of systemic sclerosis
Abstract
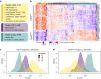
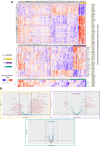
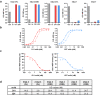
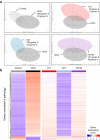
Systemic sclerosis (SSc) is an autoimmune, inflammatory and fibrotic disease with limited treatment options. Developing new therapies is therefore crucial to address patient needs. To this end, we focused on galectin-3 (Gal-3), a lectin known to be associated with several pathological processes seen in SSc. Using RNA sequencing of whole-blood samples in a cross-sectional cohort of 249 patients with SSc, Gal-3 and its interactants defined a strong transcriptomic fingerprint associated with disease severity, pulmonary and cardiac malfunctions, neutrophilia and lymphopenia. We developed new Gal-3 neutralizing monoclonal antibodies (mAb), which were then evaluated in a mouse model of hypochlorous acid (HOCl)-induced SSc. We show that two of these antibodies, D11 and E07, reduced pathological skin thickening, lung and skin collagen deposition, pulmonary macrophage content, and plasma interleukin-5 and -6 levels. Moreover, E07 changed the transcriptional profiles of HOCl-treated mice, resulting in a gene expression pattern that resembled that of control mice. Similarly, pathological pathways engaged in patients with SSc were counteracted by E07 in mice. Collectively, these findings demonstrate the translational potential of Gal-3 blockade as a therapeutic option for SSc.
© 2023. Springer Nature Limited.
Conflict of interest statement
Servier is the owner of the described therapeutic Gal-3 mAbs for which a patent application (EP22305372) has been filed. G.R., M.L.G., and J.S. are regular employees of Mabqi, a company providing contract research services and having received fees from Servier for the generation of Gal-3 neutralizing mAbs (contract number CT0081211). D.L., S.S., M.J., and T.G. are employees of the Université de Lille, which received fees from Servier for conducting evaluations in the HOCl model (contract number CT0089360). D.L. is or has been a consultant for Actelion, Boehringer Ingelheim, Octapharma, CSL Behring, Takeda, and Servier. The following authors: C.O., P.S., S.H., L.C., A.C., S.B., E.N., B.N., B.F., A.L., F.L., S.E., S.C., N.P., T.Q.N., and F.D. were regular employees of Servier at the time the research project. E.D. was a Ph.D student financed by Servier at the time of the research project. J.V. was a post-doc financed by Servier at the time of the research project.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

