Human milk oligosaccharides modulate the intestinal microbiome of healthy adults
- PMID: 37652940
- PMCID: PMC10471580
- DOI: 10.1038/s41598-023-41040-5
Human milk oligosaccharides modulate the intestinal microbiome of healthy adults
Abstract
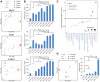
Human milk contains over 200 distinct oligosaccharides, which are critical to shaping the developing neonatal gut microbiome. To investigate whether a complex mixture of human milk oligosaccharides (HMOs) would similarly modulate the adult gut microbiome, HMO-Concentrate derived from pooled donor breast milk was administered orally to 32 healthy adults for 7 days followed by 21 days of monitoring. Fecal samples were collected for 16S rRNA gene sequencing, shotgun metagenomics, and metabolomics analyses. HMO-Concentrate induced dose-dependent Bifidobacterium expansion, reduced microbial diversity, and altered microbial gene content. Following HMO cessation, a microbial succession occurred with diverse taxonomic changes-including Bacteroides expansion-that persisted through day 28. This was associated with altered microbial gene content, shifts in serum metabolite levels, and increased circulating TGFβ and IL-10. Incubation of cultured adult microbiota with HMO-Concentrate induced dose-dependent compositional shifts that were not recapitulated by individual HMOs or defined mixtures of the 10 most abundant HMOs in HMO-Concentrate at their measured concentrations. These findings support that pooled donor HMOs can exert direct effects on adult gut microbiota and that complex mixtures including low abundance HMOs present in donor milk may be required for maximum effect.Registration: ClinicalTrials.gov NCT05516225.
© 2023. Springer Nature Limited.
Conflict of interest statement
MLL, DJR, AKS, CA, and VN are current or former (VN) employees of Prolacta Bioscience, Inc. JPJ received consulting fees from Prolacta Bioscience, Inc.
Figures






References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

