Scalable production of tissue-like vascularized liver organoids from human PSCs
- PMID: 37653039
- PMCID: PMC10545717
- DOI: 10.1038/s12276-023-01074-1
Scalable production of tissue-like vascularized liver organoids from human PSCs
Abstract
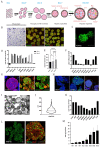
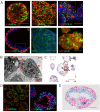
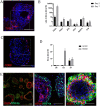
The lack of physiological parity between 2D cell culture and in vivo culture has led to the development of more organotypic models, such as organoids. Organoid models have been developed for a number of tissues, including the liver. Current organoid protocols are characterized by a reliance on extracellular matrices (ECMs), patterning in 2D culture, costly growth factors and a lack of cellular diversity, structure, and organization. Current hepatic organoid models are generally simplistic and composed of hepatocytes or cholangiocytes, rendering them less physiologically relevant compared to native tissue. We have developed an approach that does not require 2D patterning, is ECM independent, and employs small molecules to mimic embryonic liver development that produces large quantities of liver-like organoids. Using single-cell RNA sequencing and immunofluorescence, we demonstrate a liver-like cellular repertoire, a higher order cellular complexity, presenting with vascular luminal structures, and a population of resident macrophages: Kupffer cells. The organoids exhibit key liver functions, including drug metabolism, serum protein production, urea synthesis and coagulation factor production, with preserved post-translational modifications such as N-glycosylation and functionality. The organoids can be transplanted and maintained long term in mice producing human albumin. The organoids exhibit a complex cellular repertoire reflective of the organ and have de novo vascularization and liver-like function. These characteristics are a prerequisite for many applications from cellular therapy, tissue engineering, drug toxicity assessment, and disease modeling to basic developmental biology.
© 2023. The Author(s).
Conflict of interest statement
FB is partially funded by the Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 812616.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

