Adipocytes reprogram cancer cell metabolism by diverting glucose towards glycerol-3-phosphate thereby promoting metastasis
- PMID: 37653041
- PMCID: PMC12175003
- DOI: 10.1038/s42255-023-00879-8
Adipocytes reprogram cancer cell metabolism by diverting glucose towards glycerol-3-phosphate thereby promoting metastasis
Abstract
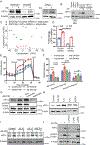
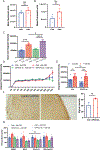
In the tumor microenvironment, adipocytes function as an alternate fuel source for cancer cells. However, whether adipocytes influence macromolecular biosynthesis in cancer cells is unknown. Here we systematically characterized the bidirectional interaction between primary human adipocytes and ovarian cancer (OvCa) cells using multi-platform metabolomics, imaging mass spectrometry, isotope tracing and gene expression analysis. We report that, in OvCa cells co-cultured with adipocytes and in metastatic tumors, a part of the glucose from glycolysis is utilized for the biosynthesis of glycerol-3-phosphate (G3P). Normoxic HIF1α protein regulates the altered flow of glucose-derived carbons in cancer cells, resulting in increased glycerophospholipids and triacylglycerol synthesis. The knockdown of HIF1α or G3P acyltransferase 3 (a regulatory enzyme of glycerophospholipid synthesis) reduced metastasis in xenograft models of OvCa. In summary, we show that, in an adipose-rich tumor microenvironment, cancer cells generate G3P as a precursor for critical membrane and signaling components, thereby promoting metastasis. Targeting biosynthetic processes specific to adipose-rich tumor microenvironments might be an effective strategy against metastasis.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
There are no potential conflicts of interest. E.L. received funding from Abbvie and Arsenal Bioscience for preclinical research studies unrelated to the submitted paper.
Figures



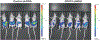







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

