Effects and plasma proteomic analysis of GLP-1RA versus CPA/EE, in combination with metformin, on overweight PCOS women: a randomized controlled trial
- PMID: 37653215
- PMCID: PMC10806039
- DOI: 10.1007/s12020-023-03487-4
Effects and plasma proteomic analysis of GLP-1RA versus CPA/EE, in combination with metformin, on overweight PCOS women: a randomized controlled trial
Abstract
Purpose: Polycystic ovary syndrome (PCOS) is characterized by reproductive dysfunctions and metabolic disorders. This study aims to compare the therapeutic effectiveness of glucagon-like peptide-1 receptor agonist (GLP-1RA) + Metformin (Met) versus cyproterone acetate/ethinylestradiol (CPA/EE) + Met in overweight PCOS women and identify potential proteomic biomarkers of disease risk in women with PCOS.
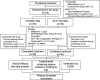
Methods: In this prospective, open-label randomized controlled trial, we recruited 60 overweight PCOS women into two groups at a 1:1 ratio to receive CPA/EE (2 mg/day: 2 mg cyproterone acetate and 35-μg ethinylestradiol,) +Met (1500 mg/day) or GLP-1 RA (liraglutide, 1.2-1.8 mg/day) +Met (1500 mg/day) for 12 weeks. The clinical effectiveness and adverse effects were evaluated, followed by plasma proteomic analysis and verification of critical biomarkers by ELISA.
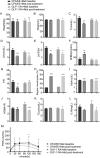
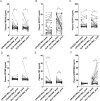
Results: Eighty(80%) patients completed the study. Both interventions improved menstrual cycle, polycystic ovaries, LH(luteinizing hormone) and HbA1c(hemoglobin A1c) levels after the 12-week treatment. GLP-1RA + Met was more effective than CPA/EE + Met in reducing body weight, BMI (Body Mass Index), and waist circumference, FBG(fasting blood glucose), AUCI(area under curve of insulin),TC (Total Cholesterol), IL-6(Interleukin-6) and improving insulin sensitivity, and ovulation in overweight women with PCOS, with acceptable short-term side effects. CPA/EE + Met was more effective in improving hyperandrogenemia, including T(total testosterone), LH, LH/FSH(Luteinizing hormone/follicle-stimulating hormone), SHBG(sex hormone-binding globulin) and FAI (free androgen index). By contract, GLP-1RA+Met group only improved LH. Plasma proteomic analysis revealed that the interventions altered proteins involved in reactive oxygen species detoxification (PRDX6, GSTO1, GSTP1, GSTM2), platelet degranulation (FN1), and the immune response (SERPINB9).
Conclusions: Both CPA/EE+Met and GLP-1RA + Met treatment improved reproductive functions in overweight PCOS women. GLP-1RA + Met was more effective than CPA/EE + Met in reducing body weight, BMI, and waist, and improving metabolism, and ovulation in overweight women with PCOS, with acceptable short-term side effects. CPA/EE + Met was more effective in reducing hyperandrogenemia. The novel plasma biomarkers PRDX6, FN1, and SERPINB9, might be indicators and targets for PCOS treatment. TRIAL REGISTRATION CLINICALTIALS.
Gov trial no: NCT03151005. Registered 12 May, 2017, https://clinicaltrials.gov/ct2/show/NCT03151005 .
Keywords: Glucagon-like Peptide-1 Receptor Agonist (GLP-1 RA); Metformin; PCOS (Polycystic Ovary Syndrome); Proteomics Analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human reproduction (Oxford, England);19(1):41−7 (2004) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

