DNA-PK and the TRF2 iDDR inhibit MRN-initiated resection at leading-end telomeres
- PMID: 37653239
- PMCID: PMC10497418
- DOI: 10.1038/s41594-023-01072-x
DNA-PK and the TRF2 iDDR inhibit MRN-initiated resection at leading-end telomeres
Abstract
Telomeres replicated by leading-strand synthesis lack the 3' overhang required for telomere protection. Surprisingly, resection of these blunt telomeres is initiated by the telomere-specific 5' exonuclease Apollo rather than the Mre11-Rad50-Nbs1 (MRN) complex, the nuclease that acts at DNA breaks. Without Apollo, leading-end telomeres undergo fusion, which, as demonstrated here, is mediated by alternative end joining. Here, we show that DNA-PK and TRF2 coordinate the repression of MRN at blunt mouse telomeres. DNA-PK represses an MRN-dependent long-range resection, while the endonuclease activity of MRN-CtIP, which could cleave DNA-PK off of blunt telomere ends, is inhibited in vitro and in vivo by the iDDR of TRF2. AlphaFold-Multimer predicts a conserved association of the iDDR with Rad50, potentially interfering with CtIP binding and MRN endonuclease activation. We propose that repression of MRN-mediated resection is a conserved aspect of telomere maintenance and represents an ancient feature of DNA-PK and the iDDR.
© 2023. The Author(s).
Conflict of interest statement
T.d.L. is a member of the scientific advisory board of Calico, San Francisco, CA, USA. The remaining authors declare no competing interests.
Figures
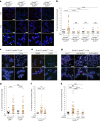



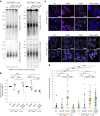
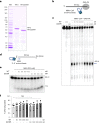



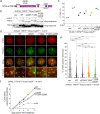



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

