Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2- advanced breast cancer receiving first-line ribociclib plus fulvestrant
- PMID: 37653397
- PMCID: PMC10469877
- DOI: 10.1186/s13058-023-01701-9
Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2- advanced breast cancer receiving first-line ribociclib plus fulvestrant
Abstract
Background: The phase III MONALEESA-3 trial included first- (1L) and second-line (2L) patients and demonstrated a significant overall survival (OS) benefit for ribociclib + fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer (ABC) in the final protocol-specified and exploratory (longer follow-up) OS analyses. At the time of these analyses, the full OS benefit of 1L ribociclib was not completely characterized because the median OS (mOS) was not reached. As CDK4/6 inhibitor (CDK4/6i) + endocrine therapy (ET) is now a preferred option for 1L HR+/HER2- ABC, we report an exploratory analysis (median follow-up, 70.8 months; 14.5 months longer than the prior analysis) to fully elucidate the OS benefit in the MONALEESA-3 1L population.
Methods: Postmenopausal patients with HR+/HER2- ABC were randomized 2:1 to 1L/2L fulvestrant + ribociclib or placebo. OS in 1L patients (de novo disease or relapse > 12 months from completion of [neo]adjuvant ET) was assessed by Cox proportional hazards model and Kaplan-Meier methods. Progression-free survival 2 (PFS2) and chemotherapy-free survival (CFS) were analyzed. MONALEESA-3 is registered with ClinicalTrials.gov (NCT02422615).
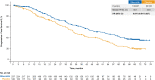
Results: At data cutoff (January 12, 2022; median follow-up time, 70.8 months), mOS was 67.6 versus 51.8 months with 1L ribociclib versus placebo (hazard ratio (HR) 0.67; 95% CI 0.50-0.90); 16.5% and 8.6% of ribociclib and placebo patients, respectively, were still receiving treatment. PFS2 (HR 0.64) and CFS (HR 0.62) favored ribociclib versus placebo. Among those who discontinued treatment, 16.7% and 35.0% on ribociclib or placebo, respectively, received a subsequent CDK4/6i. No new safety signals were observed.
Conclusions: This analysis of MONALEESA-3 reports the longest mOS thus far (67.6 months) for 1L patients in a phase III ABC trial. These results in a 1L population show that the OS benefit of ribociclib was maintained through extended follow-up, further supporting its use in HR+/HER2- ABC.
Keywords: Advanced breast cancer; CDK4/6 inhibitor; First line; Overall survival; Ribociclib.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
PN has nothing to disclose. PAF reports personal fees for advisory boards and invited speaker fees from Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, Eisai, Merck Sharp & Dohme, Lilly, Seagen, Roche, and Gilead; institutional funding from BioNTech and Cepheid; research grant from Pfizer; and personal fees for advisory boards from Pierre Fabre, Hexal, Agendia, and Sanofi Aventis. SC reports personal fees for advisory boards and institutional grants for participation in clinical trials from Novartis, Pfizer, Hoffmann-LaRoche, and Eli Lilly during the conduct of the study and outside the submitted work. GJ reports personal fees and nonfinancial support from Novartis during the conduct of the study; personal fees and nonfinancial support from Novartis, Roche, Pfizer, Lilly, Amgen, BMS, AstraZeneca, AbbVie, Daiichi Sankyo, and Seagen outside the submitted work; and nonfinancial support from MedImmune and Merck KGaA outside the submitted work. MDL reports personal fees for speaker honoraria and advisory board honoraria from Pfizer, Novartis, Roche, AstraZeneca, Eisai, Eli Lilly, and Pierre Fabre outside the submitted work and personal fees for advisory board honoraria from MSD outside the submitted work. S-AI reports research grants from AstraZeneca, Eisai, Daewoong, Pfizer, and Roche; personal fees and nonfinancial support for presenting results of clinical trial from Novartis; and personal fees from AstraZeneca, Hanmi, Pfizer, Eisai, Amgen, GSK, MSD, Roche, and Lilly outside the submitted work. KP reports personal fees for advisory boards from Novartis, AstraZeneca, Roche, and Pfizer outside the submitted work. GVB reports personal fees for advisory boards from Roche, Novartis, Eli Lilly, Seagen, AstraZeneca, Daiichi Sankyo, and MSD outside the submitted work. MM reports personal fees for speaker honoraria and honoraria for participation in advisory boards from Lilly and Pfizer; honoraria for participation in advisory boards from AstraZeneca, GlaxoSmithKline, PharmaMar, and Taiho Oncology; and research grants and honoraria for participation in advisory boards from Novartis and Roche/Genentech outside the submitted work. AN reports personal fees for consulting/advisory roles and travel/accommodation/expenses and research funding from Novartis and personal fees for consulting/advisory role from Amgen during the conduct of the study. GSS reports institutional reimbursement for patient accrual during the conduct of the study; institutional reimbursement for education and steering committee activities from Novartis; and institutional research support from Merck, AstraZeneca, Roche, and Seagen outside the submitted work. LDlCM reports personal fees from BMS, MSD/Merck, Roche, and Gilead outside the submitted work. JTB reports grants for institutional funding for doing research from AbbVie, Alliance, Argenx, Ascentage Pharma Group, AstraZeneca, Biodesix, Bio-Thera, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Hutchison, Immunomedics, Gilead, MT Group, Merck, Nektar, Pfizer, Polynoma, Seagen, Serono/EMD, Tesaro, TG Therapeutics, Daiichi Sankyo, Exact Sciences, Boehringer Ingelheim, Laekna, Novocure, Mirati Therapeutics, Tarveda Therapeutics, Sumitomo Dainippon Pharma Oncology, Elpiscience Biopharma, Takeda, Vaccinex, Vincerx Pharma, Ultimovacs, and Mersana during the conduct of the study. JPZ, YW, AC, and CW report employment with and stock ownership of Novartis. DS reports board of directors (stock) and travel expenses from BioMarin; stock ownership, research funding, and travel expenses from Pfizer; advisory board, consulting, research funding, and travel expenses from Novartis; consulting fees from Eli Lilly; and stock ownership of Amgen and Seattle Genetics outside the submitted work.
Figures



References
-
- National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology: Breast Cancer (Version 2.2022).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

