Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion
- PMID: 37653406
- PMCID: PMC10469825
- DOI: 10.1186/s12951-023-02065-0
Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion
Abstract
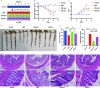
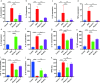
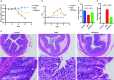
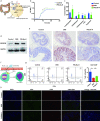
Plant-derived exosome-like nanoparticles (PDENs) have been paid great attention in the treatment of ulcerative colitis (UC). As a proof of concept, we isolated and identified Portulaca oleracea L-derived exosome-like nanoparticles (PELNs) from edible Portulaca oleracea L, which exhibited desirable nano-size (~ 160 nm) and a negative zeta potential value (-31.4 mV). Oral administration of PELNs effectively suppressed the expressions of pro-inflammatory cytokines (TNF-α, IL-6, IL-12, and IL-1β) and myeloperoxidase (MPO), increased levels of the anti-inflammatory cytokine (IL-10), and alleviated acute colitis in dextran sulfate sodium (DSS)-induced C57 mice and IL-10-/- mice. Notably, PELNs exhibited excellent stability and safety within the gastrointestinal tract and displayed specific targeting to inflamed sites in the colons of mice. Mechanistically, oral administration of PELNs played a crucial role in maintaining the diversity and balance of gut microbiota. Furthermore, PELNs treatment enhanced Lactobacillus reuteri growth and elevated indole derivative levels, which might activate the aryl-hydrocarbon receptor (AhR) in conventional CD4+ T cells. This activation downregulated Zbtb7b expression, leading to the reprogramming of conventional CD4+ T cells into double-positive CD4+CD8+T cells (DP CD4+CD8+ T cells). In conclusion, our findings highlighted the potential of orally administered PELNs as a novel, natural, and colon-targeted agent, offering a promising therapeutic approach for managing UC. Schematic illustration of therapeutic effects of oral Portulaca oleracea L -derived natural exosome-like nanoparticles (PELNs) on UC. PELNs treatment enhanced Lactobacillus reuteri growth and elevated indole derivative levels, which activate the aryl-hydrocarbon receptor (AhR) in conventional CD4+ T cells leading to downregulate the expression of Zbtb7b, reprogram of conventional CD4+ T cells into double-positive CD4+CD8+T cells (DP CD4+CD8+ T cells), and decrease the levels of pro-inflammatory cytokines.
Keywords: Double-positive CD4+CD8+T; Lactobacillus reuteri; Portulaca oleracea L-derived exosome-like nanoparticles; Ulcerative colitis.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: Ulcerative Colitis in adults. Am J Gastroenterol. 2019;114:384–413. - PubMed
-
- de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78. - PubMed
-
- Fantini MC, Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: pathogenesis and impact of current therapies. Dig Liver Dis. 2021;53:558–65. - PubMed
MeSH terms
Substances
Grants and funding
- No. 2023A1515011936 and No. 2021A1515010985/the Guangdong Basic and Applied Basic Research Foundation
- No. JCYJ20150403101028164, No. JCYC20170307100911479, No. JCYJ20190807145617113 and JCYJ20210324113802006/Technical Research and Development Project of Shenzhen
- No. JCYJ20150403101028164, No. JCYC20170307100911479, No. JCYJ20190807145617113 and JCYJ20210324113802006/Technical Research and Development Project of Shenzhen
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

