Multiparametric MRI radiomics for the differentiation of brain glial cell hyperplasia from low-grade glioma
- PMID: 37653513
- PMCID: PMC10472728
- DOI: 10.1186/s12880-023-01086-3
Multiparametric MRI radiomics for the differentiation of brain glial cell hyperplasia from low-grade glioma
Abstract
Background: Differentiating between low-grade glioma and brain glial cell hyperplasia is crucial for the customized clinical treatment of patients.
Objective: Based on multiparametric MRI imaging and clinical risk factors, a radiomics-clinical model and nomogram were constructed for the distinction of brain glial cell hyperplasia from low-grade glioma.
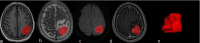
Methods: Patients with brain glial cell hyperplasia and low-grade glioma who underwent surgery at the First Affiliated Hospital of Soochow University from March 2016 to March 2022 were retrospectively included. In this study, A total of 41 patients of brain glial cell hyperplasia and 87 patients of low-grade glioma were divided into training group and validation group randomly at a ratio of 7:3. Radiomics features were extracted from T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), contrast-enhanced T1-weighted imaging (T1-enhanced). Then, LASSO, SVM, and RF models were created in order to choose a model with a greater level of efficiency for calculating each patient's Rad-score (radiomics score). The independent risk factors were identified via univariate and multivariate logistic regression analysis to filter the Rad-score and clinical risk variables in turn. A radiomics-clinical model was next built of which effectiveness was assessed.
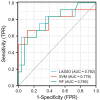
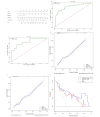
Results: Brain glial cell hyperplasia and low-grade gliomas from the 128 cases were randomly divided into 10 groups, of which 7 served as training group and 3 as validation group. The mass effect and Rad-score were two independent risk variables used in the construction of the radiomics-clinical model, and their respective AUCs for the training group and validation group were 0.847 and 0.858. The diagnostic accuracy, sensitivity, and specificity of the validation group were 0.821, 0.750, and 0.852 respectively.
Conclusion: Combining with radiomics constructed by multiparametric MRI images and clinical features, the radiomics-clinical model and nomogram that were developed to distinguish between brain glial cell hyperplasia and low-grade glioma had a good performance.
Keywords: Brain glial cell hyperplasia; Low-grade glioma; Multiparametric MRI images; Radiomics.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Glioma grading prediction using multiparametric magnetic resonance imaging-based radiomics combined with proton magnetic resonance spectroscopy and diffusion tensor imaging.Med Phys. 2022 Jul;49(7):4419-4429. doi: 10.1002/mp.15648. Epub 2022 Apr 18. Med Phys. 2022. PMID: 35366379
-
Radiomics Nomogram Building From Multiparametric MRI to Predict Grade in Patients With Glioma: A Cohort Study.J Magn Reson Imaging. 2019 Mar;49(3):825-833. doi: 10.1002/jmri.26265. Epub 2018 Sep 8. J Magn Reson Imaging. 2019. PMID: 30260592
-
The efficacy of using a multiparametric magnetic resonance imaging-based radiomics model to distinguish glioma recurrence from pseudoprogression.Magn Reson Imaging. 2024 Sep;111:168-178. doi: 10.1016/j.mri.2024.05.003. Epub 2024 May 8. Magn Reson Imaging. 2024. PMID: 38729227
-
Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma.Eur Radiol. 2020 Apr;30(4):2142-2151. doi: 10.1007/s00330-019-06548-3. Epub 2019 Dec 11. Eur Radiol. 2020. PMID: 31828414
-
High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 2: Spectroscopy, Chemical Exchange Saturation, Multiparametric Imaging, and Radiomics.Front Oncol. 2022 Feb 28;11:811425. doi: 10.3389/fonc.2021.811425. eCollection 2021. Front Oncol. 2022. PMID: 35340697 Free PMC article. Review.
Cited by
-
Current Landscape of Preclinical Models for Pediatric Gliomas: Clinical Implications and Future Directions.Cancers (Basel). 2025 Jul 2;17(13):2221. doi: 10.3390/cancers17132221. Cancers (Basel). 2025. PMID: 40647519 Free PMC article. Review.
-
Multimodal Machine Learning-Based Ductal Carcinoma in situ Prediction from Breast Fibromatosis.Cancer Manag Res. 2024 Jul 12;16:811-823. doi: 10.2147/CMAR.S467400. eCollection 2024. Cancer Manag Res. 2024. PMID: 39044747 Free PMC article.
-
Deciphering Glioblastoma: Fundamental and Novel Insights into the Biology and Therapeutic Strategies of Gliomas.Curr Issues Mol Biol. 2024 Mar 13;46(3):2402-2443. doi: 10.3390/cimb46030153. Curr Issues Mol Biol. 2024. PMID: 38534769 Free PMC article. Review.
-
A Radiomic Model for Gliomas Grade and Patient Survival Prediction.Bioengineering (Basel). 2025 Apr 24;12(5):450. doi: 10.3390/bioengineering12050450. Bioengineering (Basel). 2025. PMID: 40428069 Free PMC article.
References
-
- Shao-Feng Z, Yi-Wei H, Yang S, Si-Jie X, Jian-Ming S, Hong-Bo Y. Research based on the inflammatory response caused by the proliferation of glial cells in Alzheimer’s Disease. World J Complex Med. 2021;7(3):15–7.
-
- Biaorui S, Zhenyu L, Pingping H, Yu L. Effect of electroacupuncture on reactive astrogliosis of rats with spinal cord injury. Int J Traditional Chin Med. 2019;41(3):263–8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

