Photomanipulation of Minimal Synthetic Cells: Area Increase, Softening, and Interleaflet Coupling of Membrane Models Doped with Azobenzene-Lipid Photoswitches
- PMID: 37653602
- PMCID: PMC10625111
- DOI: 10.1002/advs.202304336
Photomanipulation of Minimal Synthetic Cells: Area Increase, Softening, and Interleaflet Coupling of Membrane Models Doped with Azobenzene-Lipid Photoswitches
Abstract
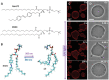
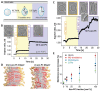
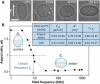
Light can effectively interrogate biological systems in a reversible and physiologically compatible manner with high spatiotemporal precision. Understanding the biophysics of photo-induced processes in bio-systems is crucial for achieving relevant clinical applications. Employing membranes doped with the photolipid azobenzene-phosphatidylcholine (azo-PC), a holistic picture of light-triggered changes in membrane kinetics, morphology, and material properties obtained from correlative studies on cell-sized vesicles, Langmuir monolayers, supported lipid bilayers, and molecular dynamics simulations is provided. Light-induced membrane area increases as high as ≈25% and a ten-fold decrease in the membrane bending rigidity is observed upon trans-to-cis azo-PC isomerization associated with membrane leaflet coupling and molecular curvature changes. Vesicle electrodeformation measurements and atomic force microscopy reveal that trans azo-PC bilayers are thicker than palmitoyl-oleoyl phosphatidylcholine (POPC) bilayers but have higher specific membrane capacitance and dielectric constant suggesting an increased ability to store electric charges across the membrane. Lastly, incubating POPC vesicles with azo-PC solutions results in the insertion of azo-PC in the membrane enabling them to become photoresponsive. All these results demonstrate that light can be used to finely manipulate the shape, mechanical and electric properties of photolipid-doped minimal cell models, and liposomal drug carriers, thus, presenting a promising therapeutic alternative for the repair of cellular disorders.
Keywords: atomic force microscopy (AFM); azo-PC; bending rigidity; giant vesicles; membrane capacitance; molecular dynamics simulations; photoswitchable lipids.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Masiero S., Lena S., Pieraccini S., Spada G. P., Angew. Chem., Int. Ed. 2008, 47, 3184; - PubMed
- b) Wang Z., Erhart P., Li T., Zhang Z.‐Y., Sampedro D., Hu Z., Wegner H. A., Brummel O., Libuda J., Nielsen M. B., Moth‐Poulsen K., Joule 2021, 5, 3116.
-
- Baigl D., Lab Chip 2012, 12, 3637. - PubMed
-
- Palacci J., Sacanna S., Vatchinsky A., Chaikin P. M., Pine D. J., J. Am. Chem. Soc. 2013, 135, 15978. - PubMed
-
- Mallick A., Roy S., Nanoscale 2018, 10, 12713. - PubMed
-
- Pacheco M., Jurado‐Sánchez B., Escarpa A., Angew. Chem., Int. Ed. 2019, 58, 18017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
