Beta vulgaris L.-A Source with a Great Potential in the Extraction of Natural Dyes Intended for the Sustainable Dyeing of Wool
- PMID: 37653849
- PMCID: PMC10222782
- DOI: 10.3390/plants12101933
Beta vulgaris L.-A Source with a Great Potential in the Extraction of Natural Dyes Intended for the Sustainable Dyeing of Wool
Abstract
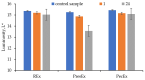
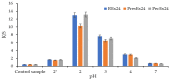
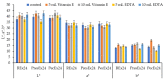
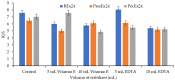
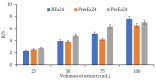
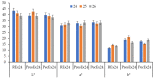
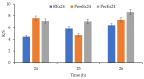
Beta vulgaris L. is a biennial plant easily accessible all over the world, rich in various biologically active compounds, especially a class of extremely bioactive pigments known as betalains. These dyes predominate in the pulp and peels of beetroot, which is why they can be valorized in food, medicine or in the textile industry. In this work, betalains extractions were carried out applying 3 sustainable options: (1) dissolving/solubilizing betalains in water; (2) extraction under pressure; (3) extraction assisted by an enzyme/pectinase. The obtained extracts were analyzed in the UV-Vis domain, which allowed their characterization by determining the total monomeric anthocyanins, color density (control), polymeric density and browning index. The HPLC-MS analysis highlighted the extracts composition. The colors characteristics were determined through CIELab measurements. The performances of these 3 extracts, during green dyeing (without mordants), were evaluated according to the color characteristics (L*, a*, b* and K/S) of the dyed wool samples under different conditions: pH, temperature, duration of dyeing and volume of extract and stabilizers (Vitamin E and EDTA). Betalains can be considered acid dyes, with a low affinity for wool, which in a pronounced acidic environment dye the wool in an intense, uniform way and with good resistance to washing and rubbing.
Keywords: betalains; decarboxylation; deprotonation; dyeing; pectinase; pressure; stabilizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Samanta A.K., Konar A. Dyeing of textiles with natural dyes. In: Kumbasar E.A., editor. Natural Dyes. InTech; Fontana, CA, USA: 2011. [(accessed on 10 August 2022)]. Available online: http://www.intechopen.com/books/natural-dyes/dyeing-of-textiles-with-nat....
-
- Restricted Substance List. [(accessed on 5 March 2023)]. Available online: https://cdn.regatta.com/attachments/restricted-substances.pdf.
-
- Padma S., Vankar D.S. New Trends in Natural Dyes for Textiles. 1st ed. Elsevier; Duxford, UK: 2019. Newer natural dyes for various textiles; pp. 1–30.
-
- Saxena S., Raja A.S.M. Natural dyes: Sources, chemistry, application and sustainability issues. In: Muthu S.S., editor. Roadmap to Sustainable Textiles and Clothing. Textile Science and Clothing Technology. Springer; Singapore: 2014. pp. 33–80.
Grants and funding
LinkOut - more resources
Full Text Sources

