Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: State of the art
- PMID: 37654433
- PMCID: PMC10465967
- DOI: 10.1016/j.jot.2023.07.005
Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: State of the art
Abstract
Background: Magnetic resonance imaging (MRI) is the dominant 3D imaging modality to quantify muscle properties in skeletal muscle disorders, in inherited and acquired muscle diseases, and in sarcopenia, in cachexia and frailty.
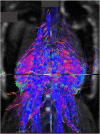
Methods: This review covers T1 weighted and Dixon sequences, introduces T2 mapping, diffusion tensor imaging (DTI) and non-proton MRI. Technical concepts, strengths, limitations and translational aspects of these techniques are discussed in detail. Examples of clinical applications are outlined. For comparison 31P-and 13C-MR Spectroscopy are also addressed.
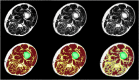
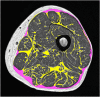
Results: MRI technology provides a rich toolset to assess muscle deterioration. In addition to classical measures such as muscle atrophy using T1 weighted imaging and fat infiltration using Dixon sequences, parameters characterizing inflammation from T2 maps, tissue sodium using non-proton MRI techniques or concentration or fiber architecture using diffusion tensor imaging may be useful for an even earlier diagnosis of the impairment of muscle quality.
Conclusion: Quantitative MRI provides new options for muscle research and clinical applications. Current limitations that also impair its more widespread use in clinical trials are lack of standardization, ambiguity of image segmentation and analysis approaches, a multitude of outcome parameters without a clear strategy which ones to use and the lack of normal data.
Keywords: Clinical trials; Diffusion tensor imaging; Dixon; Muscle MRI; Quantitative analysis; T2 mapping.
© 2023 The Authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. KE is a part time and MABE a full time employee of Clario, Inc.
Figures
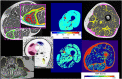
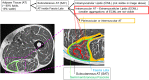
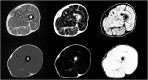
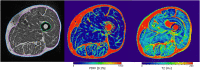




References
-
- Mercuri E., Pichiecchio A., Allsop J., Messina S., Pane M., Muntoni F. Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imag. 2007 Feb;25(2):433–440. - PubMed
-
- Smitaman E., Flores D.V., Mejia Gomez C., Pathria M.N. MR imaging of atraumatic muscle disorders. Radiographics. 2018 Mar-Apr;38(2):500–522. - PubMed
-
- Ten Dam L., van der Kooi A.J., Verhamme C., Wattjes M.P., de Visser M. Muscle imaging in inherited and acquired muscle diseases. Eur J Neurol. 2016 Apr;23(4):688–703. - PubMed
-
- Weber A., editor. Magnetic resonance imaging of skeletal musculature. Springer; Heidelberg: 2014.
Publication types
LinkOut - more resources
Full Text Sources

