Structure-Property Relationships for Nickel Aluminate Catalysts in Polyethylene Hydrogenolysis with Low Methane Selectivity
- PMID: 37654574
- PMCID: PMC10466342
- DOI: 10.1021/jacsau.3c00232
Structure-Property Relationships for Nickel Aluminate Catalysts in Polyethylene Hydrogenolysis with Low Methane Selectivity
Abstract
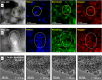
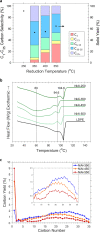
Earth-abundant metals have recently been demonstrated as cheap catalyst alternatives to scarce noble metals for polyethylene hydrogenolysis. However, high methane selectivities hinder industrial feasibility. Herein, we demonstrate that low-temperature ex-situ reduction (350 °C) of coprecipitated nickel aluminate catalysts yields a methane selectivity of <5% at moderate polymer deconstruction (25-45%). A reduction temperature up to 550 °C increases the methane selectivity nearly sevenfold. Catalyst characterization (XRD, XAS, 27Al MAS NMR, H2 TPR, XPS, and CO-IR) elucidates the complex process of Ni nanoparticle formation, and air-free XPS directly after reaction reveals tetrahedrally coordinated Ni2+ cations promote methane production. Metallic and the specific cationic Ni appear responsible for hydrogenolysis of internal and terminal C-C scissions, respectively. A structure-methane selectivity relationship is discovered to guide the design of Ni-based catalysts with low methane generation. It paves the way for discovering other structure-property relations in plastics hydrogenolysis. These catalysts are also effective for polypropylene hydrogenolysis.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- OECD . Global Plastics Outlook; 2022.
-
- Nova-Institute Bioplastics Market Development Update 2020. Eur. Bioplastics Org. 2020, 1–2. 10.1016/j.focat.2020.05.008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
