Metabolic Disorders Are Associated With Drug-Induced Liver Injury During Antituberculosis Treatment: A Multicenter Prospective Observational Cohort Study in Korea
- PMID: 37654787
- PMCID: PMC10468151
- DOI: 10.1093/ofid/ofad422
Metabolic Disorders Are Associated With Drug-Induced Liver Injury During Antituberculosis Treatment: A Multicenter Prospective Observational Cohort Study in Korea
Abstract
Background: Drug-induced liver injury (DILI) may lead to the discontinuation of antituberculosis (anti-TB) treatment (ATT). Some studies have suggested that metabolic disorders increase the risk of DILI during ATT. This study aimed to identify risk factors for DILI, particularly metabolic disorders, during ATT.
Methods: A multicenter prospective observational cohort study to evaluate adverse events during ATT was conducted in Korea from 2019 to 2021. Drug-susceptible patients with TB who had been treated with standard ATT for 6 months were included. The patients were divided into 2 groups depending on the presence of 1 or more metabolic conditions, such as insulin resistance, hypertension, obesity, and dyslipidemia. We monitored ATT-related adverse events, including DILI, and treatment outcomes. The incidence of DILI was compared between individuals with and without metabolic disorders, and related factors were evaluated.
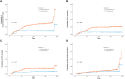
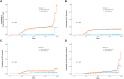
Results: Of 684 patients, 52 (7.6%) experienced DILI, and 92.9% of them had metabolic disorders. In the multivariable analyses, underlying metabolic disorders (adjusted hazard ratio [aHR], 2.85; 95% CI, 1.01-8.07) and serum albumin <3.5 g/dL (aHR, 2.26; 95% CI, 1.29-3.96) were risk factors for DILI during ATT. In the 1-month landmark analyses, metabolic disorders were linked to an elevated risk of DILI, especially significant alanine aminotransferase elevation. The treatment outcome was not affected by the presence of metabolic disorders.
Conclusions: Patients with metabolic disorders have an increased risk of ATT-induced liver injury compared with controls. The presence of metabolic disorders and hypoalbuminemia adversely affects the liver in patients with ATT.
Keywords: antitubercular agents; chemical and drug-induced liver injury; metabolic syndrome; risk factors; tuberculosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

Similar articles
-
Incidence, Clinical Features, Associated Factors and Outcomes of Intensive Phase Antituberculosis Drug Induced Liver Injury Among Patients With Tuberculosis at a Tertiary Care Hospital in Nepal: A Descriptive Cross-Sectional Study.Health Sci Rep. 2025 Apr 21;8(4):e70686. doi: 10.1002/hsr2.70686. eCollection 2025 Apr. Health Sci Rep. 2025. PMID: 40260049 Free PMC article.
-
Predicting antitubercular drug-induced liver injury and its outcome and introducing a novel scoring system.Int J Mycobacteriol. 2021 Apr-Jun;10(2):116-121. doi: 10.4103/ijmy.ijmy_15_21. Int J Mycobacteriol. 2021. PMID: 34558461
-
Predicting drug-induced liver injury from anti-tuberculous medications by early monitoring of liver tests.J Infect. 2021 Feb;82(2):240-244. doi: 10.1016/j.jinf.2020.09.038. Epub 2020 Dec 1. J Infect. 2021. PMID: 33271167
-
Antituberculous drug-induced liver injury: current perspective.Trop Gastroenterol. 2011 Jul-Sep;32(3):167-74. Trop Gastroenterol. 2011. PMID: 22332331 Review.
-
Appropriate chemopreventive strategy for anti-tubercular therapy related liver injury is unsettled: Results from a systematic review and network meta-analysis.Expert Rev Clin Pharmacol. 2020 Nov;13(11):1253-1262. doi: 10.1080/17512433.2020.1835468. Epub 2020 Oct 27. Expert Rev Clin Pharmacol. 2020. PMID: 33043729
Cited by
-
Clinical risk factors for moderate and severe antituberculosis drug-induced liver injury.Front Pharmacol. 2024 Jul 23;15:1406454. doi: 10.3389/fphar.2024.1406454. eCollection 2024. Front Pharmacol. 2024. PMID: 39108745 Free PMC article.
-
Acute liver failure from anti-tuberculosis drug-induced liver injury: An update.World J Hepatol. 2025 May 27;17(5):106618. doi: 10.4254/wjh.v17.i5.106618. World J Hepatol. 2025. PMID: 40501479 Free PMC article. Review.
-
Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI).Nutrients. 2024 Aug 24;16(17):2837. doi: 10.3390/nu16172837. Nutrients. 2024. PMID: 39275155 Free PMC article. Review.
-
Impact of pyrazinamide usage on serious adverse events in elderly tuberculosis patients: A multicenter cohort study.PLoS One. 2024 Sep 26;19(9):e0309902. doi: 10.1371/journal.pone.0309902. eCollection 2024. PLoS One. 2024. PMID: 39325726 Free PMC article.
-
Nutrition Status and Comorbidities Are Important Factors Associated With Mortality During Anti-Tuberculosis Treatment.J Korean Med Sci. 2025 May 5;40(17):e73. doi: 10.3346/jkms.2025.40.e73. J Korean Med Sci. 2025. PMID: 40329790 Free PMC article.
References
-
- Devarbhavi H, Aithal G, Treeprasertsuk S, et al. . Asia Pacific Association of Study of Liver. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol Int 2021; 15:258–82. - PubMed
-
- European Association for the Study of the Liver . EASL clinical practice guidelines: drug-induced liver injury. J Hepatol 2019; 70:1222–61. - PubMed
-
- Li X, Tang J, Mao Y. Incidence and risk factors of drug-induced liver injury. Liver Int 2022; 42:1999–2014. - PubMed
-
- World Health Organization . World Health Organization global tuberculosis report 2021. 2021. Available at: https://www.who.int/publications/digital/global-tuberculosis-report-2021.... Accessed February 2, 2023.

