Role of long non-coding RNAs in cancer: From subcellular localization to nanoparticle-mediated targeted regulation
- PMID: 37655045
- PMCID: PMC10466435
- DOI: 10.1016/j.omtn.2023.07.009
Role of long non-coding RNAs in cancer: From subcellular localization to nanoparticle-mediated targeted regulation
Abstract
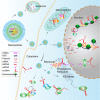
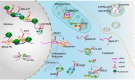
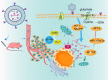
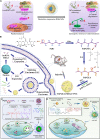
Long non-coding RNAs (lncRNAs) are a class of RNA transcripts more than 200 nucleotides in length that play crucial roles in cancer development and progression. With the rapid development of high-throughput sequencing technology, a considerable number of lncRNAs have been identified as novel biomarkers for predicting the prognosis of cancer patients and/or therapeutic targets for cancer therapy. In recent years, increasing evidence has shown that the biological functions and regulatory mechanisms of lncRNAs are closely associated with their subcellular localization. More importantly, based on the important roles of lncRNAs in regulating cancer progression (e.g., growth, therapeutic resistance, and metastasis) and the specific ability of nucleic acids (e.g., siRNA, mRNA, and DNA) to regulate the expression of any target genes, much effort has been exerted recently to develop nanoparticle (NP)-based nucleic acid delivery systems for in vivo regulation of lncRNA expression and cancer therapy. In this review, we introduce the subcellular localization and regulatory mechanisms of various functional lncRNAs in cancer and systemically summarize the recent development of NP-mediated nucleic acid delivery for targeted regulation of lncRNA expression and effective cancer therapy.
Keywords: MT: Non-coding RNAs; cancer therapy; long non-coding RNAs; nanoparticles; nucleic acid delivery; subcellular localization; targeted regulation.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

