Prevalence of PIK3CA mutations in Taiwanese patients with breast cancer: a retrospective next-generation sequencing database analysis
- PMID: 37655108
- PMCID: PMC10466395
- DOI: 10.3389/fonc.2023.1192946
Prevalence of PIK3CA mutations in Taiwanese patients with breast cancer: a retrospective next-generation sequencing database analysis
Abstract
Background: Breast cancer is the most common cancer type that affects women. In hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) is the most frequently mutated gene associated with poor prognosis. This study evaluated the frequency of PIK3CA mutations in the Taiwanese breast cancer population.
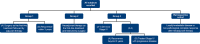
Methodology: This is a retrospective study; patient data were collected for 2 years from a next-generation sequencing database linked to electronic health records (EHRs). The primary endpoint was the regional prevalence of PIK3CA mutation. The secondary endpoints were to decipher the mutation types across breast cancer subtype, menopausal status, and time to treatment failure after everolimus (an mTOR inhibitor) or cyclin-dependent kinase 4/6 (CDK4/6) inhibitor treatment.
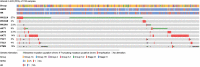
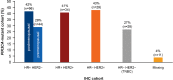
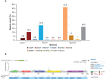
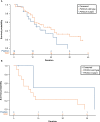
Results: PIK3CA mutations were identified in 278 of 728 patients (38%). PIK3CA mutations were reported in 43% of patients with HR-/HER2+ subtype and 42% of patients with HR+/HER2- postmenopausal status. A lower prevalence of PIK3CA mutations was observed in triple-negative (27%) and HR+/HER2- premenopausal patients (29%). The most common mutation was at exon 20 (H1047R mutation, 41.6%), followed by exon 9 (E545K mutation, 18.9% and E542K mutation, 10.3%). Among patients treated with CDK4/6 inhibitors, the median time to treatment failure was 12 months (95% CI: 7-21 months) in the PIK3CA mutation cohort and 16 months (95% CI: 11-23 months) in the PIK3CA wild-type cohort, whereas patients receiving an mTOR inhibitor reported a median time to treatment failure of 20.5 months (95% CI: 8-33 months) in the PIK3CA mutation cohort and 6 months (95% CI: 2-9 months) in the PIK3CA wild-type cohort.
Conclusion: A high frequency of PIK3CA mutations was detected in Taiwanese patients with breast cancer, which was consistent with previous studies. Early detection of PIK3CA mutations might influence therapeutic decisions, leading to better treatment outcomes.
Keywords: PIK3CA mutations; Taiwanese population; advanced breast cancer; hotspot mutations; next-generation sequencing.
Copyright © 2023 Chao, Tsai, Liu, Lien, Lin, Feng, Chen, Lai, Hsu, Lynn, Huang and Tseng.
Conflict of interest statement
Author JL is full time employee of Novartis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare his study was supported by Novartis Pharmaceuticals Corporation. The funder was involved in the study design and medical writing assistance was provided by Caamin Arora, M Pharm, from Novartis Pharmaceuticals Corporation, India.
Figures

References
-
- André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol (2021) 32(2):208–17. doi: 10.1016/j.annonc.2020.11.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

